Aluminum Anodizing
Anodizing is an electrolytic passivation process applied to a metal surface to enhance the thickness of its natural oxide layer. Specifically, aluminum anodizing involves using electrolytic passivation to strengthen the oxide layer that forms naturally on aluminum alloys.
The anodizing process forms the anode electrode of an electrical circuit. This treatment not only enhances adhesion for paint primers and glues but also increases resistance to corrosion and wear, making it more effective than untreated metals. While anodizing can be applied to various metals, including titanium, magnesium, zinc, niobium, hafnium, zirconium, and tantalum, aluminum is the most commonly anodized metal due to its susceptibility to corrosion from alloying elements such as copper and iron.
History of Aluminum Anodizing
In 1923, oxalic acid anodizing, which was based on a chromic-acid process, was first used to protect seaplane parts and architectural structures from corrosion. This method was initially patented in Japan and later adopted in Germany. In 1927, Gower and O'Brien introduced the first sulfuric acid anodizing process.
During the 1960s and 1970s, anodized aluminum became a popular material for architectural applications. However, it has since been largely replaced by more affordable alternatives like plastics and powder coatings. The phosphoric acid anodizing process emerged as a significant development more recently, though it is now primarily used as a pretreatment for adhesives and organic paints.
- Today, anodizing remains the most widely used process in the industry, with standards focusing on the coating process itself rather than the chemical methods used.
Applications for Anodizing Aluminum
Anodizing does not increase the strength of aluminum objects, but it significantly enhances their corrosion resistance, allows for dyeing, and improves lubrication and adhesion properties. Aluminum anodizing is widely used across many industries for various applications, including:
- Electronics: Provides protective outer casings for devices like cameras, MP3 players, and satellites.
- Food and Beverage: Creates durable cookware such as pots, mixers, and pans.
- Architecture: Contributes to structural integrity in areas like window frames, roofs, and exterior surface panels.
- Automotive: Used for aesthetic elements like trim, as well as protective housings for exposed parts in auto shops.
- Industrial Manufacturing: Applied to plant equipment like scales, electrolytic capacitors, and conveyors.
Anodized Aluminum Process
Anodized aluminum surfaces are harder than untreated aluminum, offering low to moderate wear resistance, which can be further improved by increasing the thickness of the anodic layer and applying suitable sealing substances. While anodic films are typically stronger and more adherent than most paints and metal platings, they are also more brittle.
The anodizing process can be categorized into three main types, with several sub-processes involved:
- Clear Anodize – Type II
- Color Anodize (such as black anodizing) – Types I, II, and III
- Titanium Anodize: Similar to aluminum anodizing, titanium anodizing is known for its variety of colors, achieved without the use of dyes.
Type I Anodizing Process
Type I chromic acid anodizing is the oldest and most widely used anodizing process. It involves an electrolytic process where the metal, with an anode attached, is immersed in a chromic acid solution, a corrosive oxidizing acid that is compatible with most aluminum alloys. As a direct current passes through the solution, the anode produces oxygen, which creates the oxide film, while a cathode produces hydrogen in the solution. A disadvantage of the Type I process is that it can reduce the aluminum thickness by 0.02 to 0.4 mils, whereas Types II and III may reduce thickness much more significantly. Additionally, the EPA restricts the use of chromic acid emissions due to its potential environmental harm. Type I also differs from Types II and III in that the DC voltage is gradually increased during the process.
Type II Anodizing Process
The Type II anodizing process utilizes sulfuric acid, a dense and corrosive acid formed from sulfur dioxide. This process is widely used for general anodizing purposes.
Type III Anodizing Process
Type III is also a sulfuric acid-based anodizing process, commonly referred to as hard anodizing. It differs from Type II by being performed at lower temperatures and with a higher electrical current density, which promotes greater anodic growth, resulting in a much harder surface.
Custom anodizing processes can be developed to meet specific needs, similar to chromate conversion processes. These methods differ based on factors like maintaining electrical conductivity or other specialized requirements.
Clear Anodizing
-
Uses for Clear Anodizing
Clear anodizing can be further enhanced with organic dyes and metallic salts to create colors like red, yellow, blue, and green for various decorative applications. These include:
- Architecture: Used in window and door frames, railings, and siding.
- Automotive: Applied to trim and housings for exposed parts.
- Printing: Used for commercial photolithography plates.
- Industrial Manufacturing: Ideal for sheet metal and extrusions such as profiles and protective cases.
- Jewelry and Artwork: Utilized for decorative and functional purposes.
The sulfuric acid anodizing process used for clear anodizing offers superior wear resistance compared to chromic acid anodizing, along with comparable corrosion and abrasion resistance. Additional benefits of clear anodizing include reduced part maintenance, lighter metal weight, environmental friendliness, and increased production efficiency.
-
Clear Anodizing Process
The clear anodizing process follows the same steps as the Type II anodizing process. In this widely used method, sulfuric acid is the electrolyte. The metal is fully immersed in sulfuric acid, a highly corrosive, oil-like acid derived from sulfur dioxide.
During the anodizing process, a direct electric current is passed through the sulfuric acid, interacting with the anodes on the surface of the material. This process forms the hard, protective oxide film. Once the anodizing process is complete, the material is removed from the acid bath and sealed with hot water to further enhance the corrosion-resistant properties of the oxide layer.
To introduce color, dyes or salts are added after immersion but before sealing, ensuring that the color becomes locked in once the sealing is complete. While color anodizing can create vibrant hues, clear anodizing allows the natural luster and finish of the metal to shine through. Although aluminum is the most commonly anodized metal, other materials like titanium, magnesium, zinc, niobium, and tantalum can also undergo clear anodizing.
Color Anodizing
-
Color Anodizing Dye Range
Color anodizing is a popular method for adding permanent color to metal products. The key advantage of this process is that the color does not simply coat the surface but is actually absorbed into the metal itself, making it resistant to scratching. Metals like titanium, zinc, magnesium, and tantalum tend to take color well, though aluminum and titanium are the most commonly anodized due to their hardness and versatility.
The anodizing process involves dyeing the oxide surface of the metal before the final sealing step, which is particularly useful when the exterior appearance is important. A wide variety of colors are available through color anodizing, ranging from light to dark shades. However, lighter colors can be more challenging to produce on certain alloys.
Color anodizing is a favored technique because it allows manufacturers to produce products in many color options. However, there are some limitations, such as the potential for certain colors to fade more quickly than others.
Using a chemical mixture like ferric ammonium oxalate on anodized aluminum can produce black and gold dyes. These inorganic dyes are more lightfast and durable than some organic colors, which, although varied, tend to fade more over time. Metallic hues, though limited in range, remain brighter for longer compared to organic dyes and are typically used in architectural applications.
The Process for Color Anodizing
The dyeing process varies depending on the type of dye used and the metal being anodized. Some metals, such as anodized titanium, naturally produce color without the need for additional dyes. The hue of anodized titanium depends on the amount of electrical current used during the anodizing process.
After a metal has been dyed, it is sealed with hot water or steam, often combined with nickel acetate, which converts the oxide layer into its hydrated form. This sealing process helps reduce color bleeding and enhances the corrosion resistance of the metal. Whether the color is part of the anodizing process or added afterward, it contributes to the high demand for anodized products due to their durability and aesthetic appeal.
Titanium Anodizing
Titanium is primarily anodized for use in jewelry and decorative items. It is frequently employed in dental implants and is also used in art, body piercing jewelry, wedding rings, and costume jewelry. The most common form of color anodization involves applying organic and chemical dyes after the anodizing process. During anodizing, the metal's surface undergoes a transformation that increases the pore size of its oxide layer, allowing it to absorb and retain color more effectively.
The resulting color depends on the thickness of the oxide layer, as the interference of light reflecting off both the oxide surface and the underlying metal creates different hues. This unique process produces vibrant and durable colors that remain stable over time.
Magnesium Anodizing
Anodized magnesium is commonly used as a primer for paint and can be sealed with substances like oil or wax. Although zinc is seldom anodized, the International Lead Zinc Organization has developed a process that creates a hard, corrosion-resistant anodized zinc with an olive-green hue.
Aluminum Anodizing Images, Diagrams and Visual Concepts
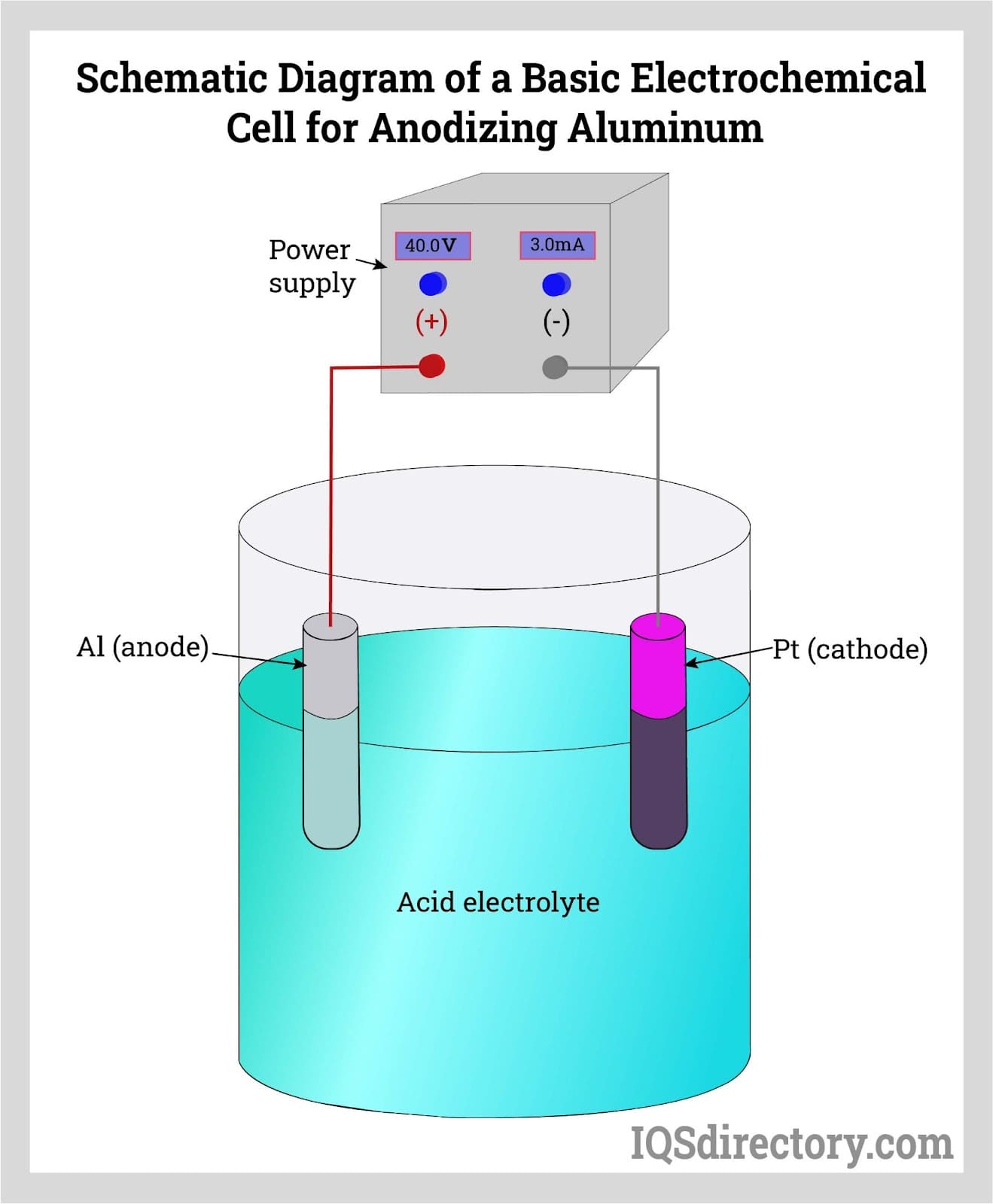 Anodizing, an electrolytic passivation process which increases the thickness of the natural oxide film on surface which is durable, stable, and corrosion resistance..
Anodizing, an electrolytic passivation process which increases the thickness of the natural oxide film on surface which is durable, stable, and corrosion resistance..
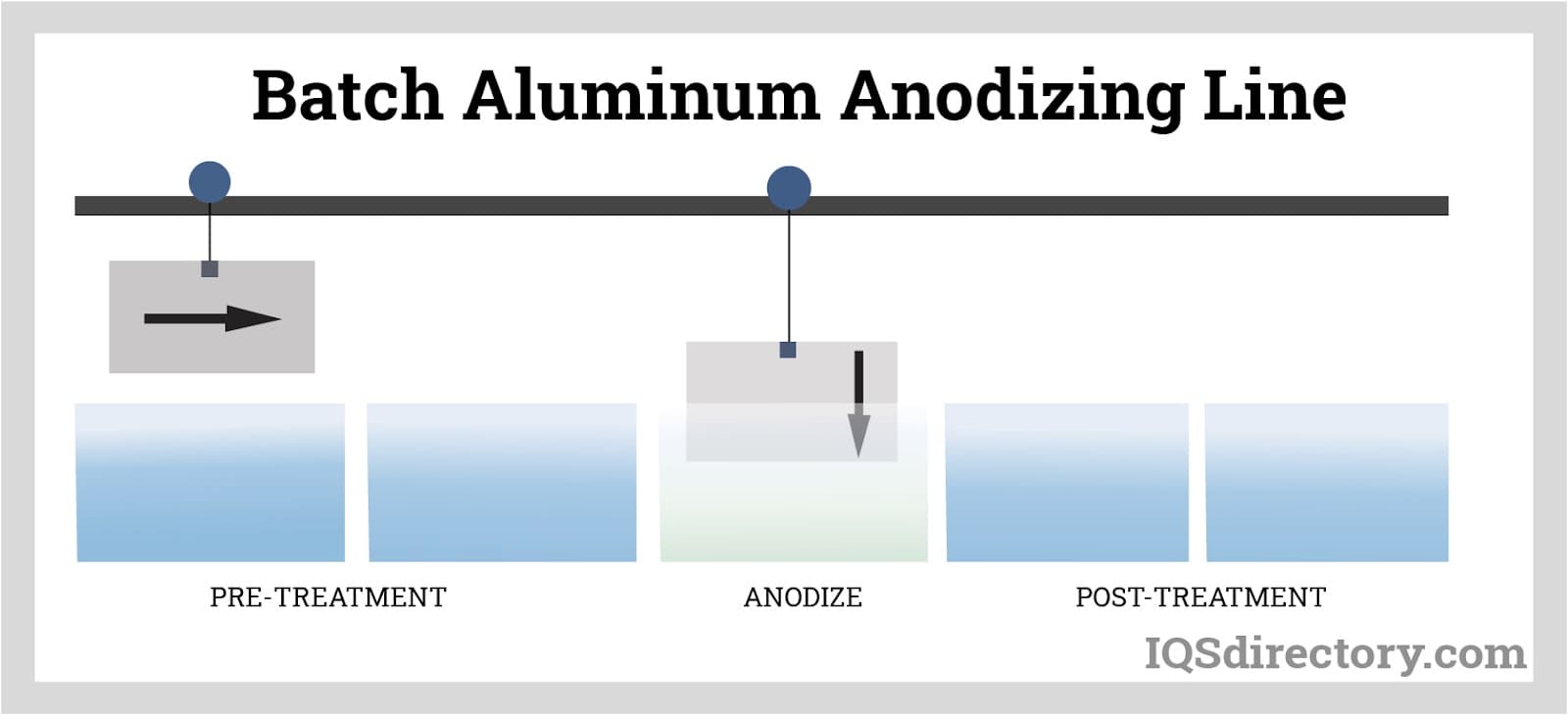 Batch anodizing, the parts are placed in a rack and immersed in a series of baths.
Batch anodizing, the parts are placed in a rack and immersed in a series of baths.
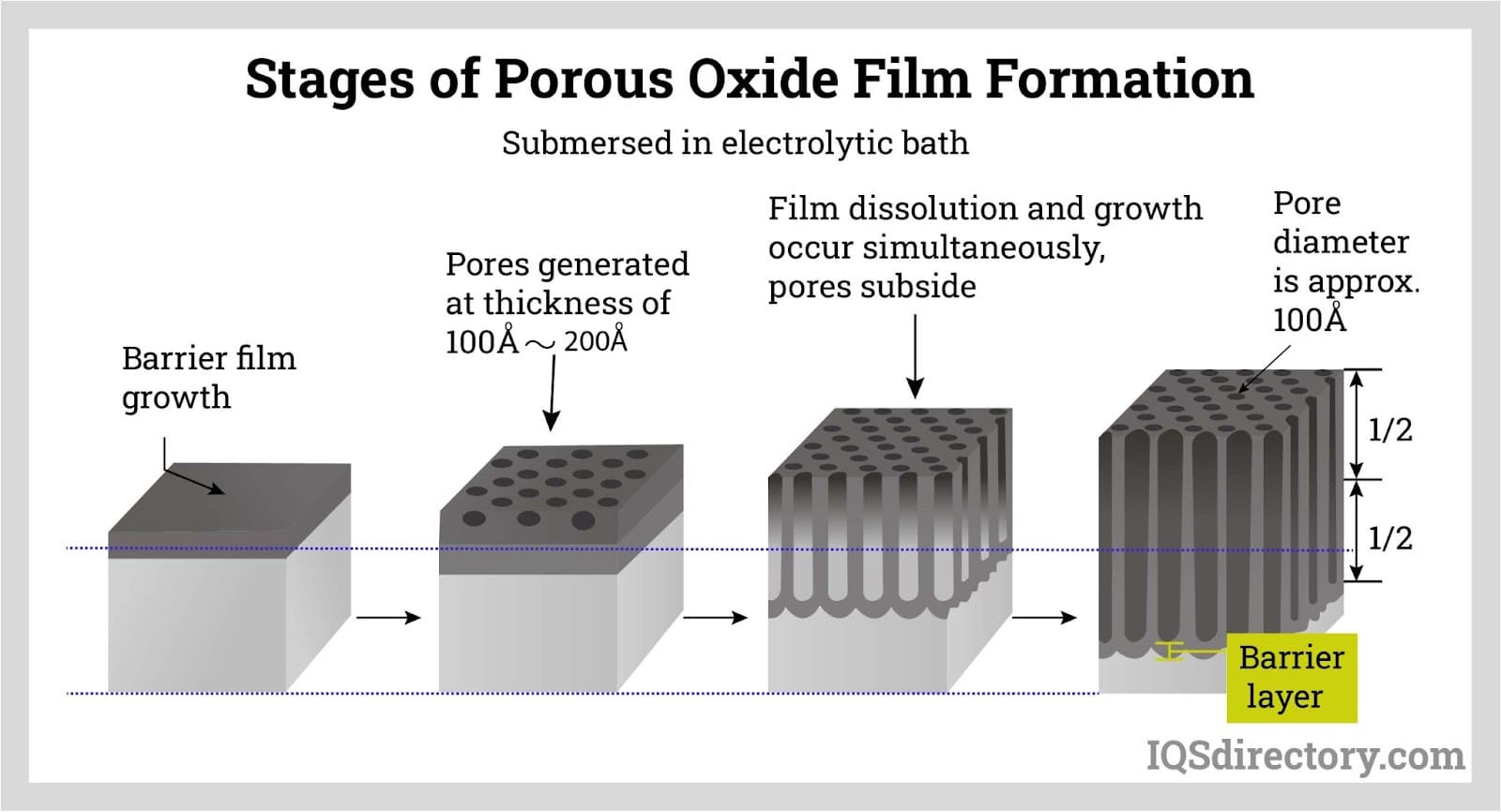 A porous oxide film is formed on the surface when grown in a dilute acidic solution and the thickness is proportional to the electrolysis time and voltage.
A porous oxide film is formed on the surface when grown in a dilute acidic solution and the thickness is proportional to the electrolysis time and voltage.
 Dye or pigment fills the porous aluminum oxide film formed by anodized making the coating is durable and cannot be removed off the surface.
Dye or pigment fills the porous aluminum oxide film formed by anodized making the coating is durable and cannot be removed off the surface.
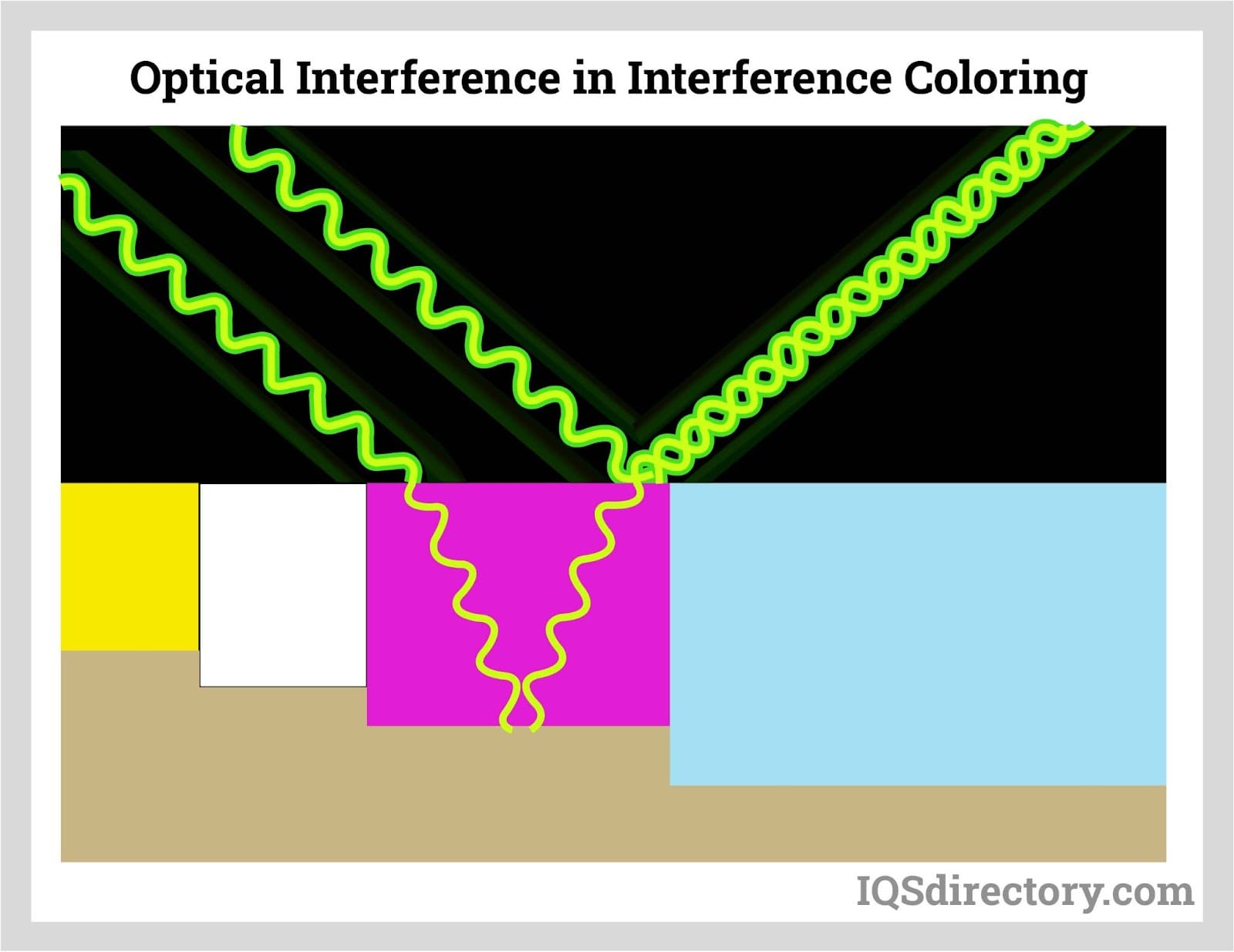 Interference coloring, the oxide film pores bases are enlarged to deposit more metallic ions electrolytically to produce light-fast colors.
Interference coloring, the oxide film pores bases are enlarged to deposit more metallic ions electrolytically to produce light-fast colors.
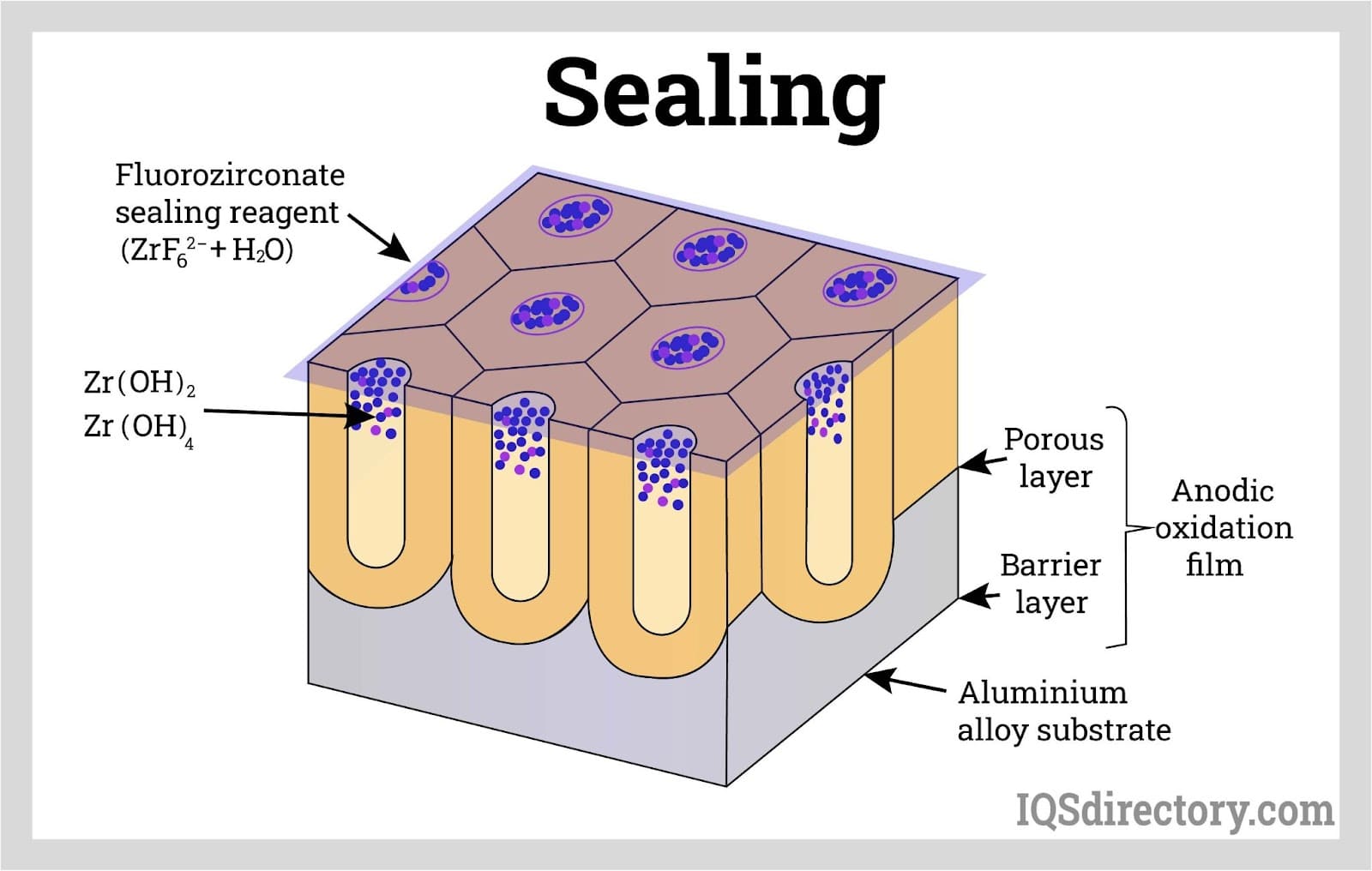 Sealing locks the absorbed dye, lubricant, or adhesive on the porous film which protects the porous film from corrosion, staining, and absorbing unwanted molecules.
Sealing locks the absorbed dye, lubricant, or adhesive on the porous film which protects the porous film from corrosion, staining, and absorbing unwanted molecules.
 Anodizing creates a stable aluminum oxide layer fully integrated with the underlying aluminum substrate.
Anodizing creates a stable aluminum oxide layer fully integrated with the underlying aluminum substrate.
Aluminum Anodizing Types
-
Anodizers
Service providers that perform the anodizing process, enhancing metals with improved wear and corrosion resistance.
Anodized Aluminum
Aluminum that has undergone an electrolytic process to enhance corrosion and wear resistance, addressing the high vulnerability of untreated aluminum alloys to corrosion.
Anodized Metal
A metallic element that has been treated through the electrolytic passivation process, known as anodizing, which forms a protective layer of oxidation on the metal's surface.
Anodizing
A technique used to coat the exterior of a metal with a protective film through an electrolytic process.
Black Anodizing
A process that coats the surface of anodized metal with a protective black dye.
Bright Dip Anodizing
A pretreatment step before anodizing, where the metal is submerged in a bath of acids to achieve a bright, shiny finish.
Chromate Conversion
A process that provides corrosion resistance and makes the aluminum surface electrically conductive, creating an ideal surface for applying paints, powder coatings, and adhesives.
Clear Anodizing
A process that pre-oxidizes the aluminum surface, creating a clear and uniform finish.
Color Anodizing
The process of applying a colored coat to the exterior of a metal.
Custom Anodizing
A specialized anodizing process where a small number of parts are uniquely anodized to achieve application-specific characteristics, such as desired hardness or color.
Hard Anodizing
A variation of anodizing that produces thicker and denser anodic films, resulting in dark tones that limit coloring options.
Sulfuric Acid Anodizing
A method that applies a thin, tightly bonded coating of aluminum oxide to the aluminum surface, providing corrosion resistance, aesthetic appeal, and electrical non-conductivity.
Impregnation
The process of filling internal cracks, voids, and corroded pockets in materials like castings and powdered metal parts to prevent leaks.
Titanium Anodizing
Most commonly used in the jewelry industry, titanium anodizing offers a high resistance to skin allergies compared to other metals.
Variations of the Aluminum Anodizing Process
Aluminum anodizing processes share the basic principles of converting the metal surface into aluminum oxide. Unlike applied coatings or electroplating, anodizing is an electrochemical conversion process that enhances the metal’s surface durability. Although aluminum is the most commonly anodized metal, other materials such as titanium, magnesium, and zinc can also undergo anodizing.
In addition to the more common materials, anodized niobium and anodized tantalum are also used, particularly in decorative applications like jewelry and commemorative coins, similar to anodized titanium. The thickness of the oxide film on these metals depends on the voltage applied during the anodizing process.
Among the metals suited for anodizing, aluminum is the most widely used due to its superior response to the anodizing process, making it the top choice in industries requiring enhanced corrosion resistance and durability.
Aluminum Anodizing Companies and Suppliers
IQS Directory offers a comprehensive list of aluminum anodizing companies and suppliers. Browse our website to find top aluminum anodizing companies, complete with detailed product descriptions and rollover ads.
Discover companies that specialize in designing and providing aluminum anodizing services tailored to your specifications. You can easily contact these companies through our convenient request for quote form. Each company profile includes essential details such as website links, company information, locations, phone numbers, product videos, and additional product details. You’ll also have access to customer reviews and the latest product news articles.
Whether you're seeking aluminum anodizing services, information on the anodizing process, aluminum anodizing equipment, or custom anodizing solutions, our directory is your go-to resource.
Aluminum Anodizing Terms
-
Abrasion
The process of using friction to wear away or grind a surface.
Activation
The process of making a metal surface chemically active.
Alloy
A material composed of two or more elements, with at least one being a metal, that exhibits metallic properties.
Alumina
An intermediate step in aluminum production derived from bauxite.
Anodizing Sheet
A sheet with suitable metallurgical characteristics and surface quality, designed for the creation of protective and decorative films through anodic oxidation.
Bath
The chemical solution in which the anodizing process takes place.
Brazing
A metal-joining process where a molten filler metal flows between the parts to be joined.
Chemical Film
The application of a chromate conversion coating on aluminum to enhance its corrosion resistance.
Corrosion
The gradual chemical or electrochemical degradation of a material, often due to environmental exposure.
Cryolite
A white mineral used in the production of aluminum.
Deburring
The process of removing burrs, sharp edges, or fins from metal surfaces through mechanical, chemical, or electrochemical methods.
Edging
The process of refining or shaping metal edges through rolling, filling, or drawing.
Hardener
An alloy containing aluminum and at least one other metal, used to modify molten aluminum.
Impurities
Unwanted elements found in aluminum compounds.
Inhibitor
A substance used to slow down or reduce the rate of a chemical or electrochemical reaction, often used to control corrosion or pickling.
Melting Point
The temperature at which a metal transitions from solid to liquid.
Passivation
The process of making a chemically active metal surface passive or non-reactive.
Plating
A method of coating one material with a thin layer of another metal.
Pores
Microscopic openings in an anodized surface, leading to microscopic tubes. Despite being porous, anodized surfaces are dense and hard.
Rack
An electrically conductive device used to hold parts during anodizing, designed in various sizes and shapes to accommodate different items.
Refined Aluminum
Aluminum that has been purified to a very high degree.
Smelt
The process of melting or fusing ore to extract or purify the metal it contains.
Striation
Long, uneven lines caused by inconsistent coating during the anodizing process.
Ultimate Strength
The maximum stress a material can withstand before failure.

