Aluminized Steel

Aluminized steels are steels that have been hot-dip coated with pure aluminum or aluminum-silicon alloys. This hot-dip coating process is termed hot-dip aluminizing (HAD)...
Please fill out the following form to submit a Request for Quote to any of the following companies listed on
This article will take an in-depth look at nickel metal and its uses.
The article will cover topics such as:

This segment delves into the essence of nickel metal, detailing its production processes and diverse applications.
Metals, renowned for their malleability, ductility, and superb thermal and electrical conductivity, are categorized into five groups, with nickel fitting into the transition metals category. Nickel is a naturally occurring metal with a shiny, silvery-white appearance and a subtle golden tinge. It has an atomic mass of 58.71, an atomic number of 28, and is symbolized by Ni. Nickel includes five stable isotopes, staying solid at standard room temperature, with a melting point of 1455°C and a boiling point of 2730°C. Known for its ferromagnetism, hardness, and resistance to corrosion and rust, nickel is ductile. This elemental metal occurs naturally, necessitating extraction through mining.

Nickel is extracted via extractive metallurgy, a technique that involves isolating and purifying the chosen metal from its ore for greater purity. Ores, natural rocks found deep within the Earth's crust, contain valuable minerals meant for profitable mining, refining, and selling. Typically, ores are metal-rich. Nickel's key ores fall into two categories: laterites and magmatic sulfides.
Nickel originates primarily from two ore deposit types: laterites, primarily containing nickel-dense limonite and garnierite, and magmatic sulfides with pentlandite as the principal ore mineral.
Metal is retrieved from ore via extractive metallurgy, with pyrometallurgical extraction being the most commonly used, utilizing high temperatures for chemical reactions. Laterite ores are mostly obtained through open-pit mining, extracting ore from extensive, open pits, while sulfide ores, often coupled with copper, are mined underground. Equipment used for mining laterite ores includes heavy shovels, draglines, and front-end loaders.
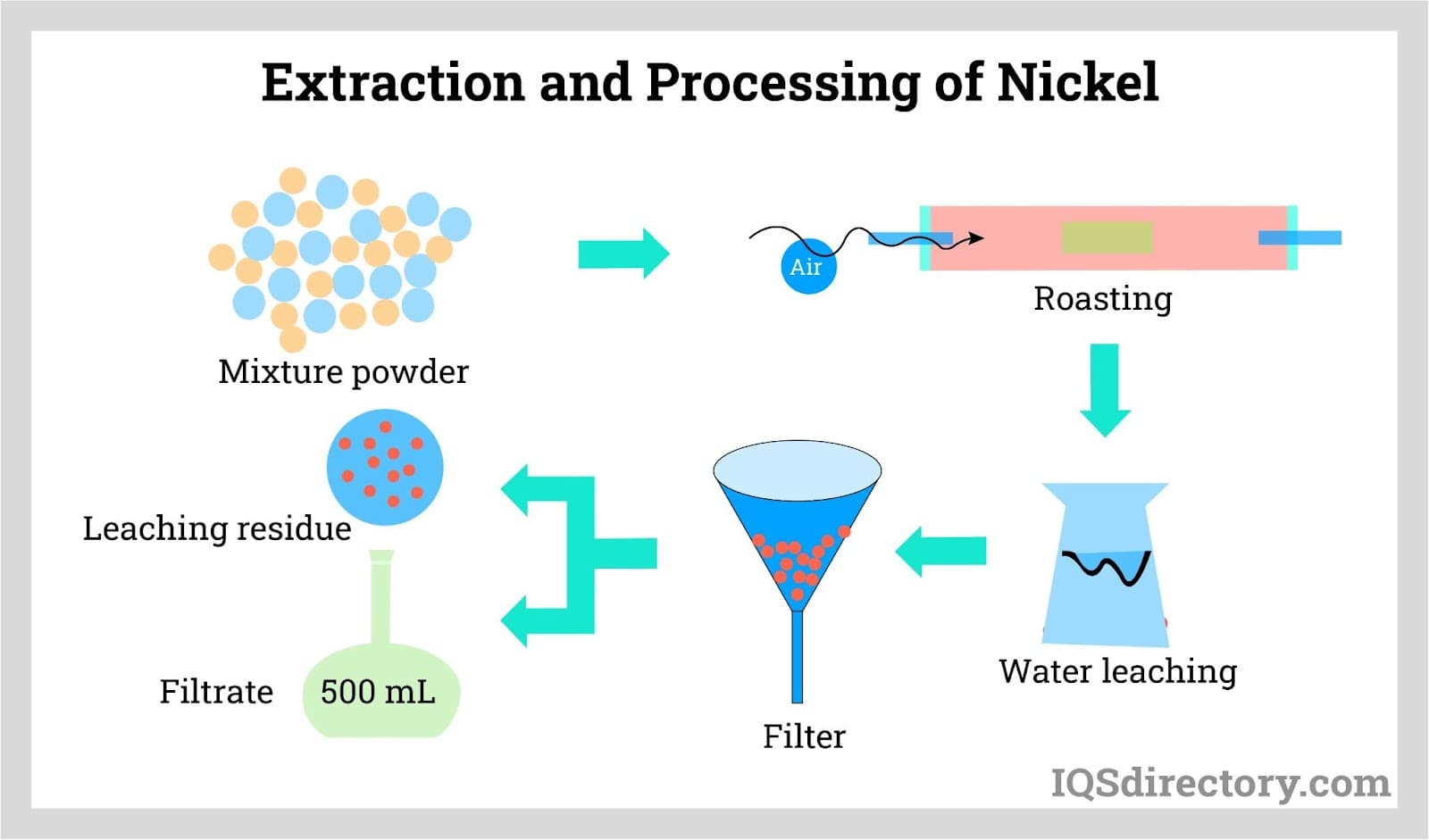
Post-mining, sulfide ores are crushed and ground, separating nickel from waste via selective flotation. This process mixes the ore with reagents and uses mechanical and pneumatic devices to create air bubbles, with the lighter sulfide particles attaching to the bubbles and rising to the surface.
Typically, the collected materials contain 6-12% nickel. Magnetic separators, leveraging the magnetic qualities of some nickel sulfides, may be used alongside or instead of flotation. Waste often undergoes secondary cleaning before disposal. For sulfide ores with nearly equal copper and nickel ratios, a second flotation step is necessary to produce a low-nickel copper concentrate and separate nickel concentrate.
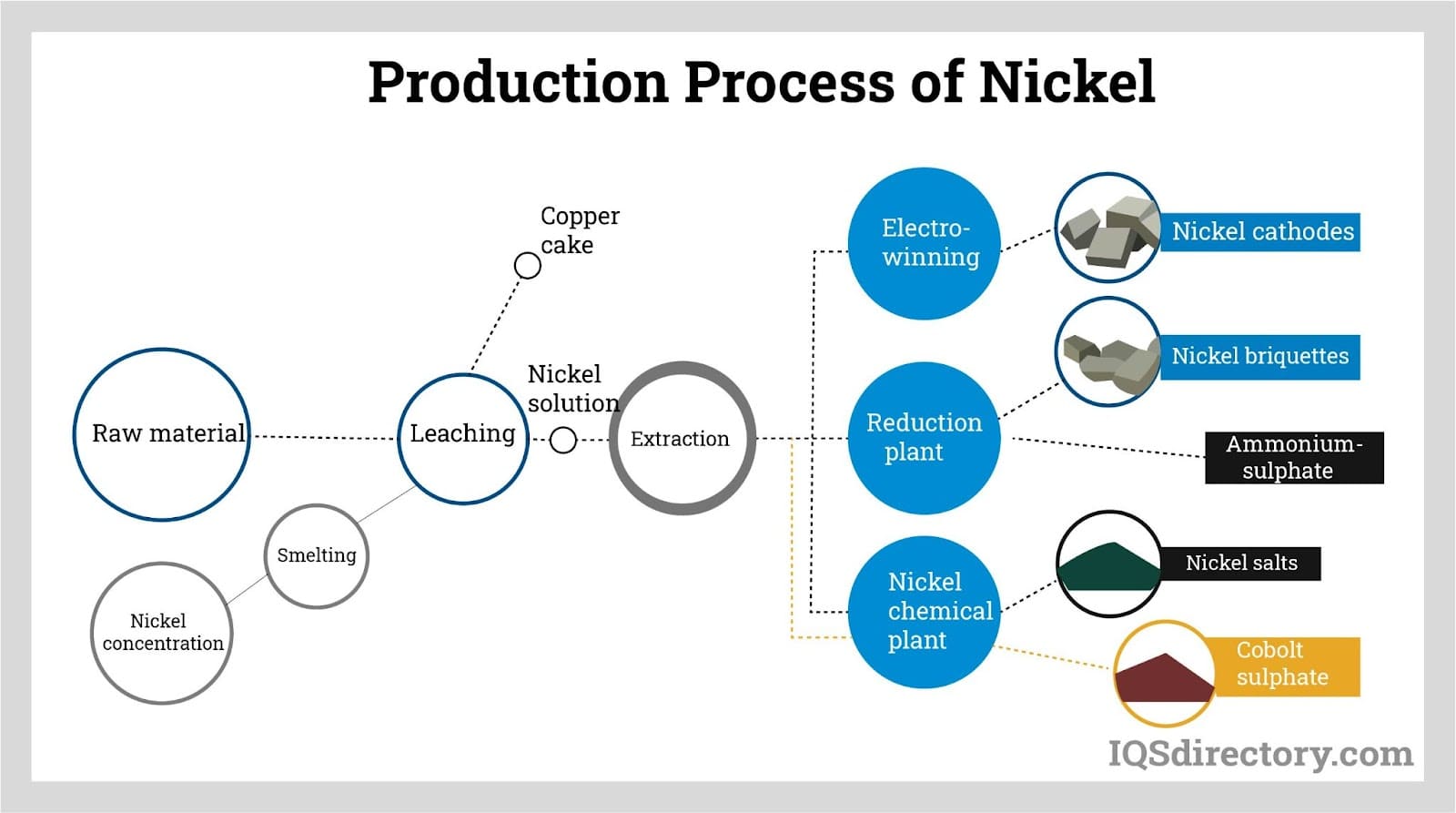
Nickel concentrates are subjected to leaching with sulfuric acid or ammonia, or alternatively dried and processed by flash smelting techniques, with flash and electric smelting being most common. In these methods, preheated oxygen is introduced into a furnace with the ore, oxidizing iron and sulfide, resulting in a nickel content of 25-45%.
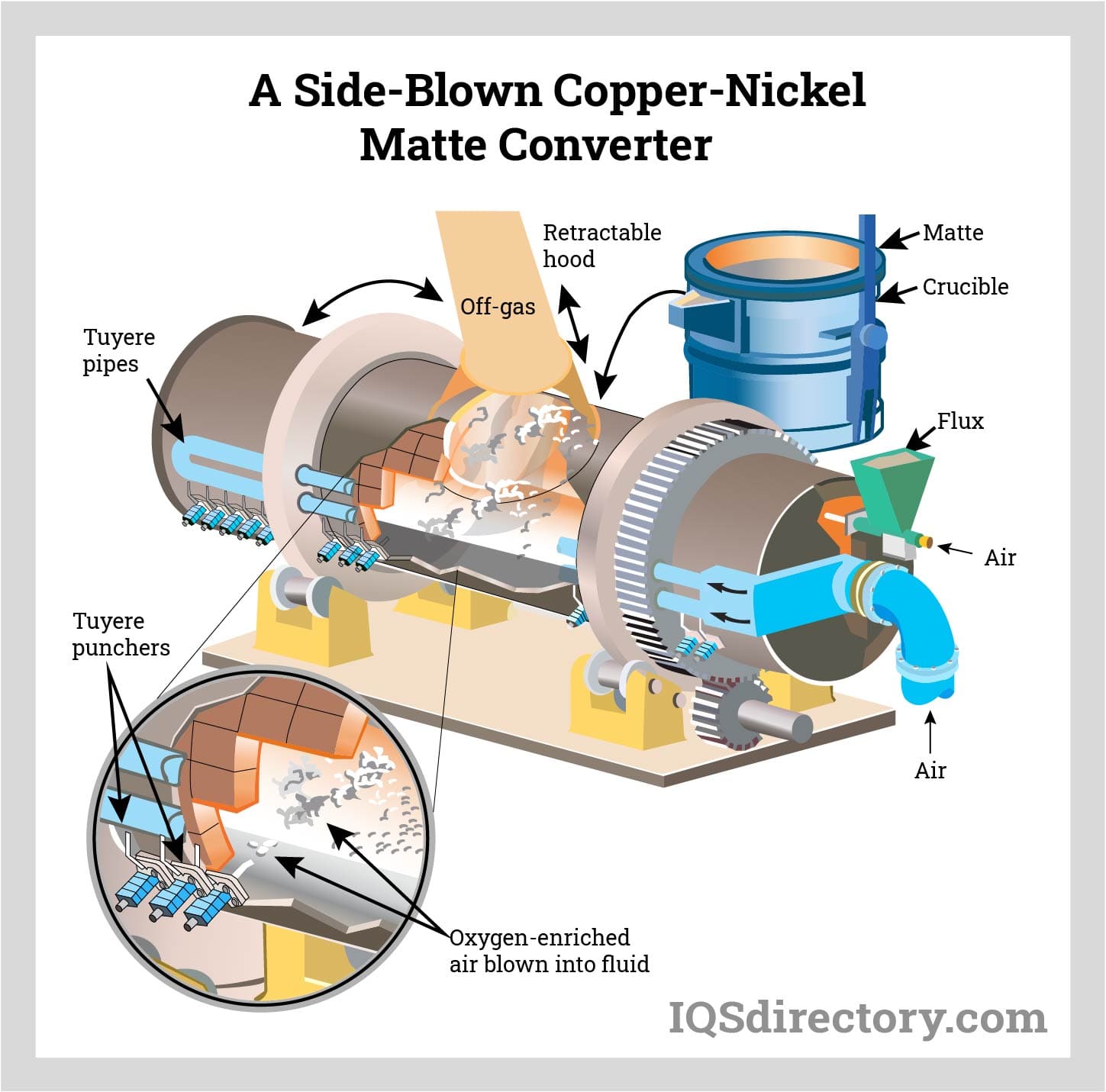
The developed nickel-iron sulfide is termed matte. In the final processing phase, oxygen is added to the molten mix, eliminating remaining iron and sulfide. This forms an oxide, reacting with silica flux to create slag, yielding nickel matte with approximately 70-75% nickel.
The entire refining process occurs within a rotating converter. Part of the energy required for smelting sulfide ores is supplied by oxygen reactions with iron and sulfur in the ore.
Extracting nickel from laterite ores, free of sulfur but high in moisture as water and hydroxides, differs from sulfide ores as it requires more energy to achieve drying and smelting due to lower reaction temperatures. Large kiln furnaces dry the ore by removing moisture.
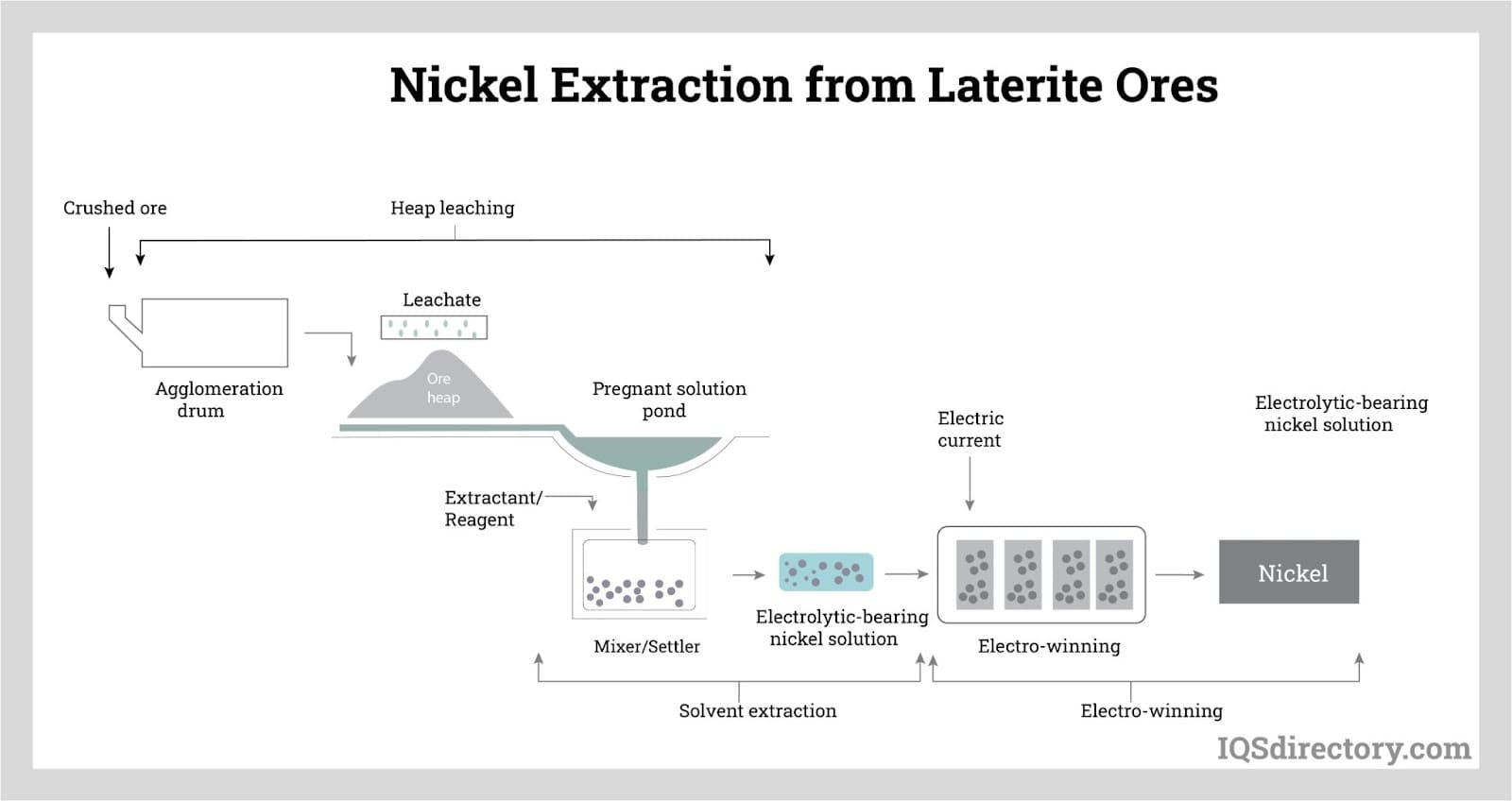
The produced nickel oxide is then reduced to nickel metal, typically in electric furnaces with 45 to 50 megavolt-amperes capacity, operating between 1360°C and 1610°C.
Post-extraction and processing, nickel matte usually contains about 75% nickel, which through refining can reach up to 95%. Methods include ammonia pressure leaching, where hydrogen reduction recovers nickel from the solution and transforms sulfur into ammonium sulfate, usable as fertilizer.
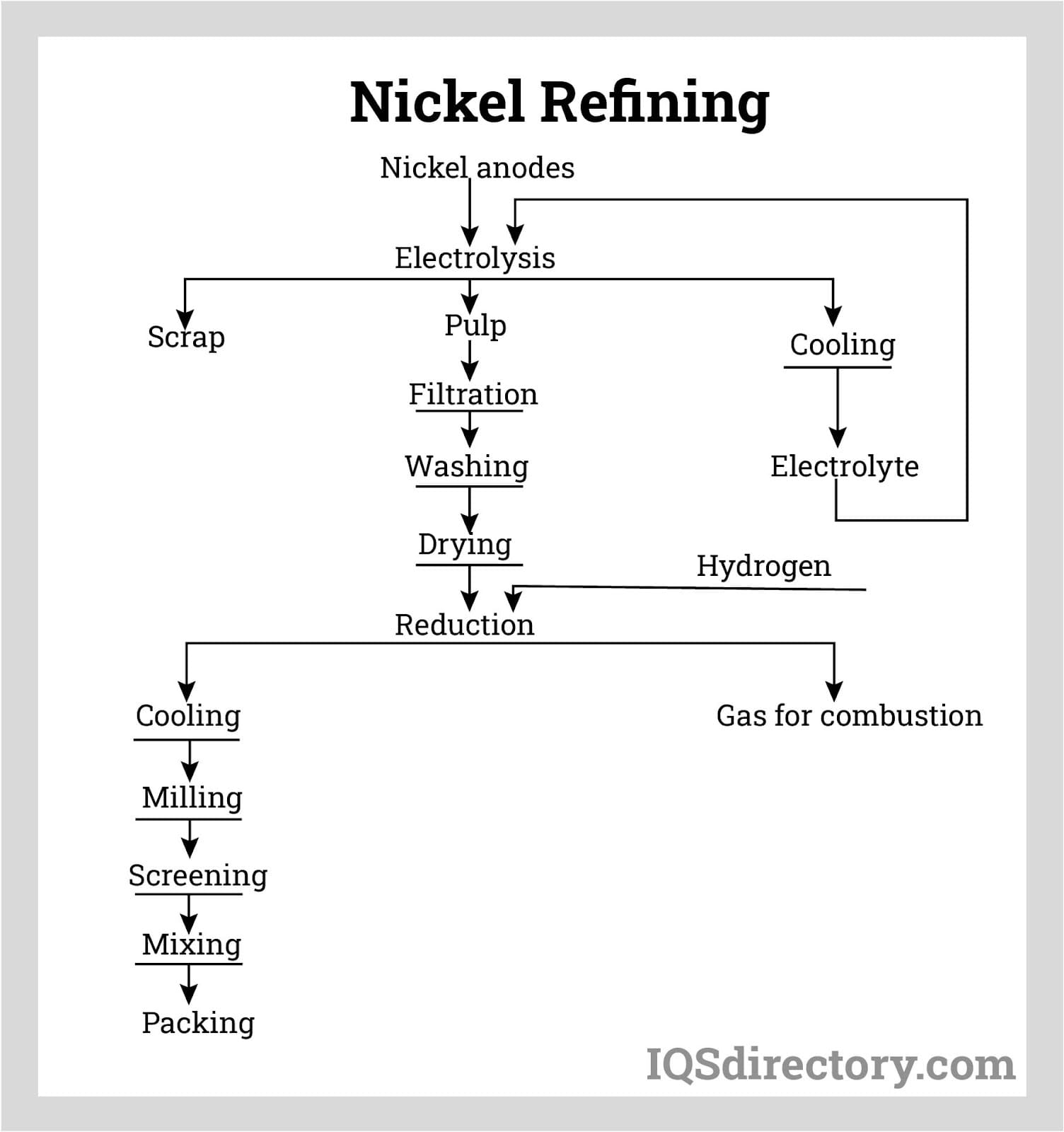
Other refinement processes incorporate matte roasting to produce high-grade nickel oxides, treated with a pressure leach. The subsequent solution undergoes further refinement via electrorefining and carbonyl refining. Electrorefining uses an electrolytic cell to achieve high-purity nickel, with diaphragm compartments preventing impurity transfer from anode to cathode. Carbonyl refining passes matte through carbon monoxide, resulting in nickel and iron carbonyls.
Refined nickel transforms into various shapes, such as bars, rods, plates, sheets, and tubing. Several methods detailed below facilitate these transformations:
This less common method for shaping nickel involves melting it at high temperatures and forming it by pushing molten nickel through a die, creating sheets, bars, rods, and tubing.

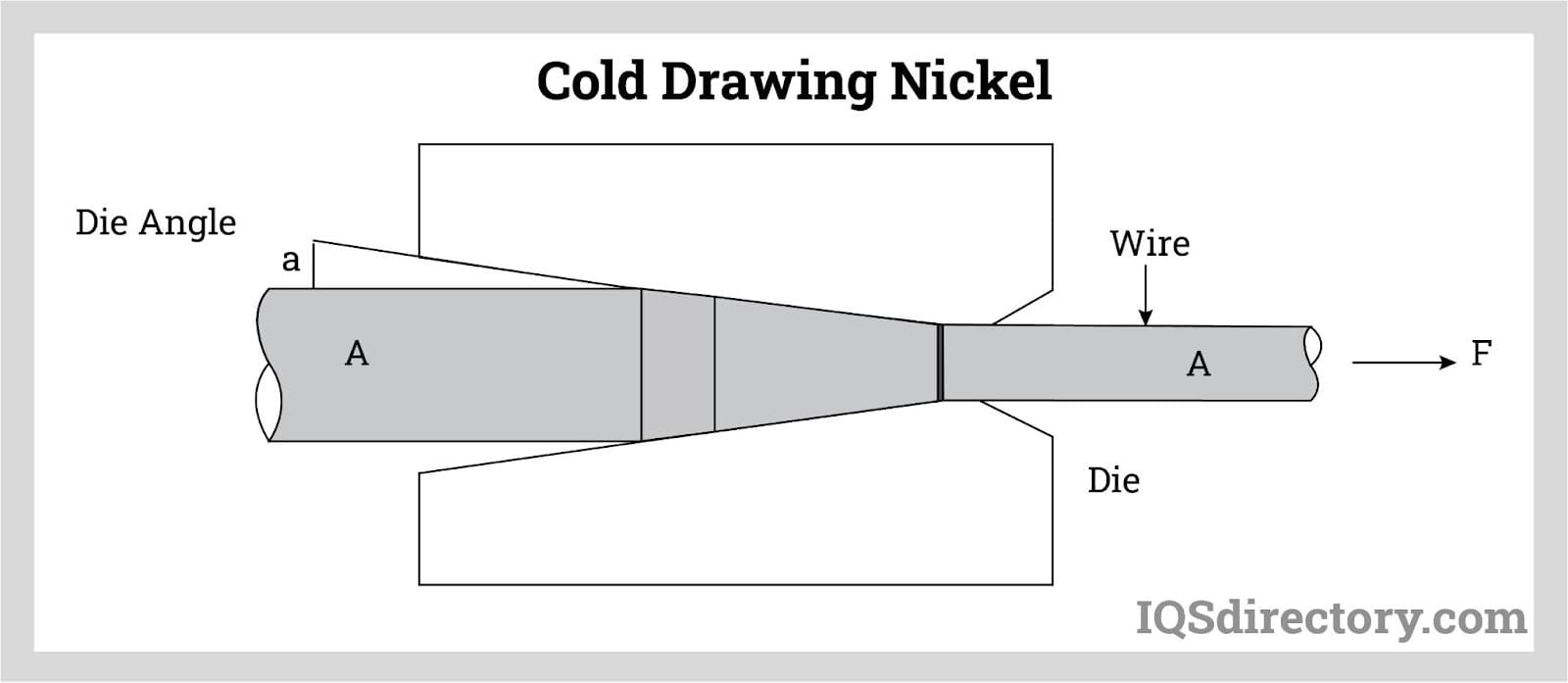
This method is more frequently used than extrusion for shaping nickel, pulling the nickel tube or wire through progressively smaller dies without applying heat, reducing its diameter, and commonly used for manufacturing nickel tubes and wires.
Employing a chemical reducing agent in an aqueous solution, this method catalytically reduces nickel ions, depositing them consistently in size and shape without electricity, and is widely used for shaping nickel.
When sourcing nickel, buyers and manufacturers must weigh numerous factors to ensure it fits their needs. Critical aspects include environmental exposure like rain and heat, the stress it will endure, intended uses, and dimensional requirements. Additionally, selecting the right supplier is crucial. Both parties should engage in detailed discussions with multiple suppliers to secure optimal pricing, lead times, secondary services, delivery protocols, and comprehensive customer support.
Nickel’s unique combination of physical and chemical properties, such as corrosion resistance, high strength, and excellent temperature stability, make it an essential material for manufacturing a wide range of nickel alloys. These specialty alloys are formed by combining nickel with other elements—including chromium, cobalt, copper, iron, and molybdenum—to optimize performance for industrial, engineering, and electrochemical applications. One of the most common and widely used nickel alloys is stainless steel, which primarily consists of iron, 18 percent chromium, and 8 percent nickel. Beyond stainless steel, nickel-based alloys and pure nickel products are integral to critical industries such as aerospace, marine, energy, oil and gas, chemical processing, electronics, automotive, and medical devices.
Nickel alloys and forms can be categorized into the following main types:
Some representative alloys and physical forms of nickel are described below. Each nickel type discussed falls under a broader category of nickel alloys listed above. Understanding these forms will help users, buyers, and engineers select the optimal material for their specific manufacturing, fabrication, and project requirements.
Brushed nickel is a finished nickel product created with a wire brush or similar abrasive surface, imparting a distinctive textured pattern while preserving a lustrous shine. As a nickel finish, it is valued for its resistance to corrosion and its ability to hide fingerprints and water spots, making it a go-to choice for interior fixtures. While brushed nickel remains durable in damp environments, it can develop a milky white tarnish over time due to oxidation and exposure to cleaning agents.

Fortunately, any tarnishing or discoloration can be easily removed with mild cleaners or a solution of water and white vinegar, restoring the original metallic appearance.
Brushed nickel is commonly used in producing:
Nickel casting alloys are created by melting combinations of nickel with other metals and pouring them into precision molds. This method enables the production of intricate shapes and components that exhibit excellent resistance to high temperatures, corrosion, and wear—essential qualities for many industrial and engineering applications. Nickel-based casting alloys, such as Inconel and Hastelloy, are especially prominent in aerospace turbine blades, energy-sector equipment, and harsh chemical environments where mechanical integrity at elevated temperatures is critical.

When selecting casting alloys, factors like thermal conductivity, oxidation resistance, and mechanical strength should be considered to ensure optimal performance.
Cupro nickel, or cupronickel, is a widely used copper-nickel alloy that also contains small amounts of iron and manganese. With copper making up 60 to 90 percent of the composition and nickel as the secondary element, cupro nickel stands out for its silver-like appearance and remarkable resistance to seawater corrosion, biofouling, and stress cracking. These properties make it a preferred choice in marine engineering, desalination plants, and shipbuilding industries. The alloy also offers good thermal conductivity, high ductility, and robust tensile strength, ensuring reliability under tough service conditions.

Cupronickel’s typical applications include:
Hastelloy refers to a group of high-performance, corrosion-resistant nickel-based alloys renowned for their ability to withstand aggressive acids, chlorides, and other harsh chemicals in industrial environments. These alloys provide exceptional resistance to pitting, crevice corrosion, and stress-corrosion cracking and are frequently specified for chemical processing, pollution control, and chemical storage tank manufacturing. Each specific Hastelloy composition is engineered to address unique corrosive challenges and thermal conditions.

The following are types of Hastelloy nickel-based alloys:
Inconel is a family of nickel-chromium-based superalloys recognized for their unmatched corrosion resistance, oxidation resistance, and exceptional performance under extreme temperatures and mechanical stresses. These properties make Inconel one of the top choices for components exposed to intensely challenging environments—such as turbine engines, petrochemical plants, and nuclear reactors—where conventional alloys fail. Inconel alloys are renowned for their ability to maintain strength, resist scaling, and prevent stress-corrosion cracking even at temperatures exceeding 700°C. However, these alloys can be challenging to machine due to their strength, sometimes requiring specialized tooling or processes.

Several types of Inconel alloys are available, including:
Invar is a nickel-iron alloy celebrated for its exceptionally low coefficient of thermal expansion (CTE). Composed of 36% nickel and 64% iron, Invar demonstrates minimal dimensional changes when subjected to temperature fluctuations, making it highly desirable for applications where precision is critical. The alloy’s stable performance has cemented its role in scientific instruments, aerospace tooling, and other fields where tight tolerances are required.

Primary applications for Invar nickel alloys include:
Kovar is a specialized alloy composed of iron, nickel, cobalt, and small quantities of manganese, silicon, and carbon. It features a precisely matched coefficient of thermal expansion to certain types of borosilicate glass, making it the leading solution for hermetic glass-to-metal seals. Kovar’s superior sealing properties, combined with excellent dimensional stability, make it indispensable in the electronics, aerospace, and scientific instrumentation industries.

Common applications include:
Monel is a distinguished family of nickel-copper alloys, typically containing 52 to 67% nickel and the remainder mostly copper plus trace levels of manganese, iron, carbon, and silicon. With superb corrosion resistance—especially in marine and chemical environments—Monel alloys are also prized for their strength, ductility, and ability to resist various acids and alkalis. Monel metals maintain integrity in both fresh and saltwater, as well as in harsh chemical process pipelines, making them a preferred choice for valves, fasteners, and pump components in offshore oil and chemical processing industries.

Common uses of monel include:
The most common Monel alloys include:
Nichrome is a nickel-chromium alloy widely recognized for its superior resistance to both high electrical loads and extreme temperatures. Exhibiting a silvery-gray appearance, nichrome forms a stable, adherent layer of chromium oxide when heated, providing remarkable oxidation resistance. Its unique properties make nichrome the industry standard for resistance wires, electrical heating elements, and temperature control devices in household and industrial appliances—including toasters, hair dryers, space heaters, and kilns.

Additional uses include bridgewires in the explosives and pyrotechnics industries, electric matches, model rocket igniters, advanced ceramics manufacturing, flame testing, and motorcycle mufflers where heat and corrosion resistance are required.
Nickel 200 refers to commercially pure wrought nickel with a purity of approximately 99.6%, containing only trace amounts of iron, manganese, silicon, copper, carbon, and sulfur. This high-purity material is highly ductile, offers excellent mechanical strength, and demonstrates outstanding resistance to caustic alkalis, acids, and neutral salts, as well as excellent thermal and electrical conductivity. Nickel 200 is the preferred choice for harsh chemical environments and high-performance applications demanding purity, integrity, and corrosion resistance.

Applications include:
Nickel rods and bars are solid, straight forms of nickel available in various shapes, including round, square, hexagonal, and triangular profiles. These products, manufactured from pure nickel or nickel-alloyed materials, provide high strength, thermal stability, and resistance to corrosive environments. Nickel bars and rods are commonly utilized in power generation plants for steam turbines, in aerospace applications for gas turbine components, in marine engineering, and for chemical processing hardware.

Various types of nickel bars include nickel hex bar, pure nickel alloy round bar, ASTM B160 nickel alloy rod, and nickel alloy square bar. They are available in multiple standards and can be tailored for specific end-use engineering and fabrication projects.
Nickel plates are flat, thick sections of rolled nickel or nickel alloys, engineered to deliver strength, durability, and corrosion resistance in demanding structural environments. These plates are critical in the construction of buildings, bridges, industrial machinery, and for components that require significant load-bearing capability paired with superior resistance to chemical attack and environmental degradation.
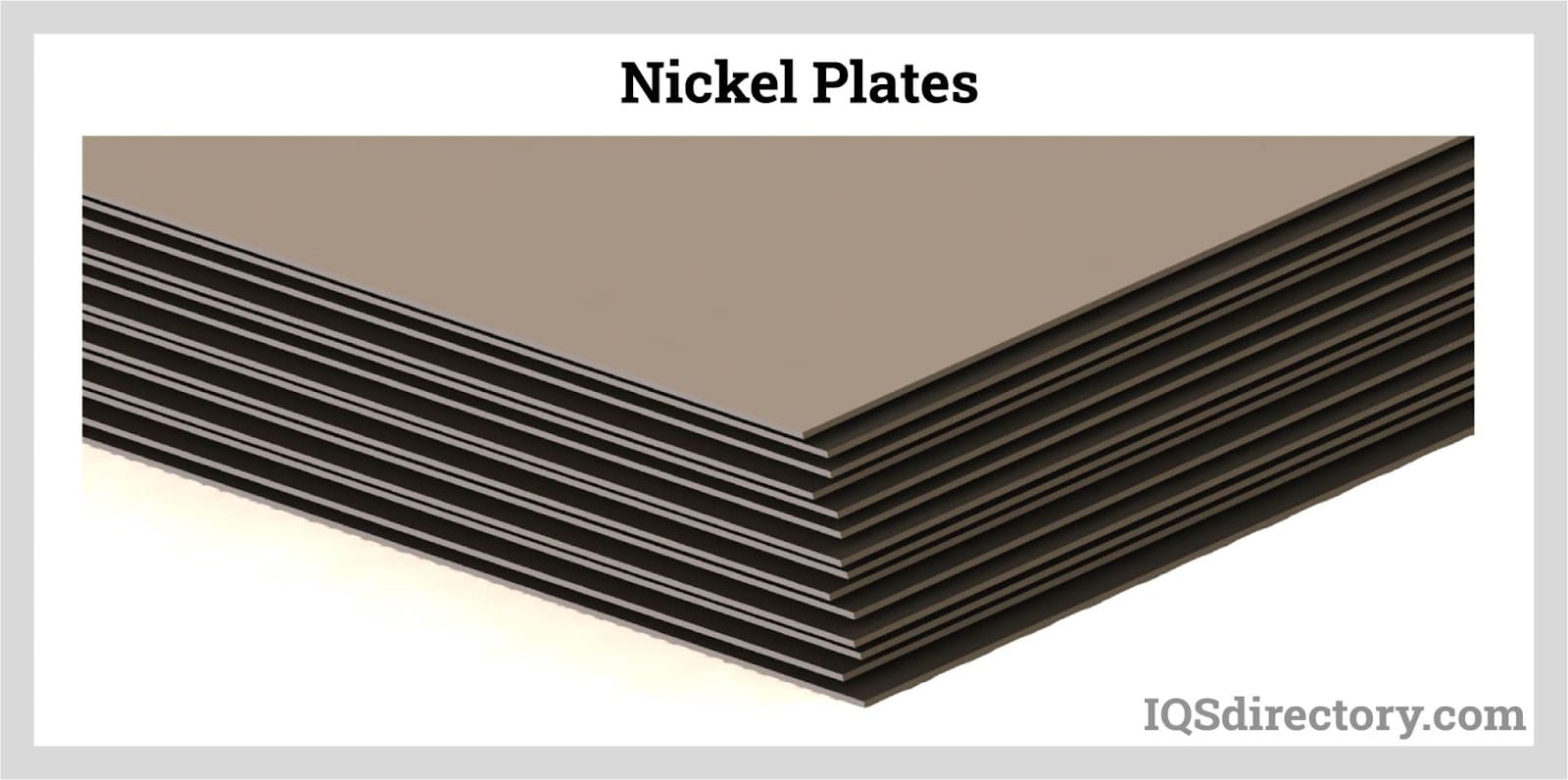
Nickel sheet is a thin, flat material created from pure nickel or nickel alloy, produced through processes such as rolling, annealing, or precision cutting. With a uniform thickness that can be customized for project-specific requirements, nickel sheets deliver outstanding weldability, malleability, thermal conductivity, and electrical conductivity. Their superior resistance to oxidation and corrosion makes nickel sheet ideal for parts used in harsh environments or where precise fabrication is needed.

Nickel sheets are used in power generation, marine engineering, electronics manufacturing, battery technology, and the aerospace industry, where their durability and performance are critical to reliability and safety.
Nickel tubing refers to seamless or welded nickel pipes and tubes, which can be manufactured in cylindrical or rectangular shapes. Nickel tubing is key for transporting aggressive fluids and gases, serving as vital components in chemical plants, petrochemical refineries, desalination systems, and high-temperature heat exchanger networks. Nickel’s outstanding formability, workability, and resistance to pitting, corrosion, and chemical attack ensures maximum longevity even in harsh service environments.

Typical nickel tube applications also include instrumentation, hydraulic lines, pumped gas systems, laboratory equipment, medical devices, and electronics manufacturing, demonstrating the alloy's versatility and performance across industries.
Permalloy is a magnetic nickel-iron alloy, typically containing 80% nickel and 20% iron. Known for its extremely high magnetic permeability and low coercivity, permalloy is especially suitable for magnetic shielding, transformers, and inductive core material in sensitive electronic and electromechanical applications. The alloy’s ability to efficiently redirect and block external magnetic fields enhances the precision of medical imaging, sensors, and high-fidelity audio equipment.

With excellent electrical resistivity and stable performance under varying operating frequencies, permalloy plays a crucial role in reducing electromagnetic interference (EMI) and enhancing signal clarity, making it indispensable for modern electronics and electrotechnical devices.
This chapter will explore the various applications and advantages of nickel metal.
As previously mentioned, nickel is a versatile metal with numerous applications. These uses are summarized in the list below:
Nickel is fully recyclable and plays a crucial role in building construction and in equipment used in power and communications industries, among others. Its durability, corrosion resistance, and hygienic properties make it valuable in the medical field. Pure nickel is rarely used on its own; it is usually alloyed with other metals to enhance ductility and strength at high temperatures.
Nickel's resistance to high temperatures reduces corrosion, allowing it to be used for extended periods without replacement. This makes it suitable for applications such as jet engines, offshore installations, and power generation facilities, where it endures extreme temperature conditions. Additionally, nickel is a key element in the stainless steel industry.
Despite the many beneficial applications and favorable properties of nickel and its alloys, there are several drawbacks associated with these materials, which are outlined below:
Nickel finishes, which are often electroplated onto metals like copper or brass, may tarnish over time due to exposure to chemicals, oils, cleaning products, acidic foods, and other substances. Harsh weather conditions can also contribute to tarnishing. To maintain nickel-plated items, use a mild soap and a soft cloth for cleaning. A water and vinegar solution can help remove dirt and oils, while bleach-based cleaners and abrasives should be avoided. Although nickel products are corrosion-resistant and do not rust, they require proper care to keep their appearance.
Nickel is a naturally occurring metallic element which belongs to the metal category known as transition metals. It has two types of ores namely: laterites and magmatic sulfide ores. An ore is a naturally occurring rock which contains one or more minerals that can be extracted, refined and then sold at a profit. The nickel is mined and extracted from the ores using two different methods. Open cut mining, a surface mining method, is used to mine the laterite ore. Laterite ores contain large amounts of water as moisture and hydroxides as a result a large amount of energy has to be used in the drying process and removal of the chemically bound water. Sulfide ores are extracted using underground mining techniques. The ore is crushed and selective floating is used to get some of the nickel from the ore and remove the waste. Magnetic separators are sometimes used as well. Both extraction and refinement of the two ores result in nickel matte, a substance which is a nickel-iron sulfide. Further refinement of the nickel matte can result in nickel with a purity of up to 95%.
Secondary manufacturing processes include casting, molding, separating, and forming. Nickel can be machined into a variety of shapes through processes such as nickel extrusion, cold drawing and Electroless nickel plating. The latter being the most common way to shape nickel. Nickel can be shaped into bars and rods, sheets, plates, tubes and many other shapes. The main use of nickel is in the making of nickel alloys.
Nickel alloys can be classified into the following categories namely: nickel-titanium alloys, nickel-chromium-cobalt alloys, nickel-chromium-molybdenum alloys, nickel-chromium-iron alloys, nickel-chromium alloys, nickel-molybdenum alloys, nickel-copper alloys, nickel-iron alloys, and wrought nickel. Nickel alloys all have different characteristics that make them suitable for a variety of applications.
Nickel is 100% recyclable and has many uses in the power, communications, marine, oil, and medical industries however it has its drawbacks. The most apparent drawback comes from the fact that mining is the only method that can be used to obtain the nickel ore of which mining is harmful to the environment. Furthermore, extracting nickel from laterite ore is expensive as a large amount of energy is required for the drying and smelting process. However nickel and nickel alloys remain an integral part of human civilization as products made from these substances are durable and can last for decades.

Aluminized steels are steels that have been hot-dip coated with pure aluminum or aluminum-silicon alloys. This hot-dip coating process is termed hot-dip aluminizing (HAD)...

The term "aluminum coil" describes aluminum that has been flattened into sheets where their width is significantly higher than their thickness and then "coiled" into a roll. Stacks of individual aluminum sheets are difficult to...
Aluminum piping and tubing is silvery-white, soft, and ductile. The metal belongs to the boron group. Aluminum is the third most abundant element present on earth. Aluminum has low density. When exposed...
Beryllium Copper is a versatile copper alloy that is valued for its high strength and hardness, combined with good electrical and thermal conductivity. It is a non-ferrous, non-magnetic, and non-sparking metal alloy...

A variety of copper-zinc alloys are referred to together as brass. Different ratios of brass and zinc can be used to create alloys, which produce materials with various mechanical, corrosion, and thermal properties...
Copper is a ductile, malleable, and reddish-gold metal with the capacity to effectively conduct heat and electricity. Brass and bronze, two commonly used alloys, are created when copper is combined with...

The copper sheet is a highly malleable and workable metal with outstanding electrical and thermal conductivity and corrosion resistance. Copper (Cu) is a reddish, very ductile metal that belongs to Group 11 of the periodic table...

Stainless steel grade 304 is an austenite stainless steel that is the most widely used and versatile of the various grades of stainless steel. It is a part of the T300 series stainless steels with...

Stainless steel is a type of steel alloy containing a minimum of 10.5% chromium. Chromium imparts corrosion resistance to the metal. Corrosion resistance is achieved by creating a thin film of metal...

Stainless steel grades each consist of carbon, iron, 10.5%-30% chromium, nickel, molybdenum, and other alloying elements. It is a popular metal used in various products, tools, equipment, and structures that serve in many industrial, commercial, and domestic applications...

Steel service centers are companies that specialize in procuring steel directly from mills and manufacturers and supplying them to the customers. They are fundamental to the steel supply chain...

Stainless steel can be fabricated using any of the traditional forming and shaping methods. Austenitic stainless steel can be rolled, spun, deep drawn, cold forged, hot forged, or stippled using force and stress...

Stainless steel tubing is a multifaceted product that is commonly utilized in structural applications. Stainless steel tubing diameters and variations vary greatly based on the application requirements and are...

Titanium metal, with the symbol Ti, is the ninth most abundant element in the earth‘s crust. It does not occur in large deposits, yet small amounts of titanium are found in almost every rock...

Tungsten is a rare naturally occurring chemical element on earth. It is known to be one of the toughest metals on the earth. It is usually a tin white or a steel gray metal. Tungsten is common for its high tensile...
Aluminum is the most abundant metal on the Earth’s crust, but it rarely exists as an elemental form. Aluminum and its alloys are valued because of their low density and high strength-to-weight ratio, durability, and corrosion resistance...