Aluminized Steel

Aluminized steels are steels that have been hot-dip coated with pure aluminum or aluminum-silicon alloys. This hot-dip coating process is termed hot-dip aluminizing (HAD)...
Please fill out the following form to submit a Request for Quote to any of the following companies listed on
This article will take an in-depth look at Tungsten metal.
The article will look at topics such as:

This section delves into the various aspects of Tungsten metal, examining its natural occurrences, manufacturing processes, and notable properties.
Tungsten is a commonly available, silvery-gray metal known for its impressive hardness. It exhibits outstanding tensile strength and can withstand extremely high temperatures, with a melting point of 6191°F (3421.7°C) and a boiling point of 10220°F (5660°C). Tungsten, identified by the atomic number 74, is part of the transition metals group in the periodic table.

Tungsten is naturally found in minerals like wolframite and scheelite. Wolframite, recognized as an iron-manganese tungstate with the formula (FeMn)WO4, is a solid solution of ferberite (FeWO4) and hubnerite (MnWO4). Scheelite, which is calcium tungstate with the formula CaWO4, is another important source. Various tungsten minerals differ in rarity and economic importance, with some being moderately common and others extremely rare with little economic value.

Below are some critical terms affiliated with tungsten metal, covering its applications and descriptions of tungsten alloys.
An alloy is produced by combining two or more elements to harness the properties of each metal, resulting in optimal performance. Noteworthy tungsten alloys include tungsten carbide (a mixture of tungsten and carbon), Hastelloy (which combines nickel, chrome, molybdenum, and tungsten), and various tungsten nickel alloys, to name a few.
The brittleness of a material indicates its propensity to fracture under stress. Pure tungsten tends to be vulnerable to cracking, leading to its alloying with other metals to boost durability. For instance, combining tungsten with carbon can significantly enhance its strength, making it suitable for applications that would otherwise be impractical for pure tungsten.
A filament is a thin metal wire used in bulbs, adept at converting electricity to light and enduring high temperatures without melting or snapping.
The melting point defines the temperature at which tungsten transitions to a liquid state. It has an exceptionally high melting point of 3,422°C (6,192°F).
Superalloys are metals noted for their high tensile strength, corrosion resistance, and elevated melting points.
In engineering contexts, tensile strength refers to the extreme stress level beyond which a metal deforms or breaks.
The alternative name, "wolfram," comes from tungsten's chief ore, wolframite, which is the primary resource for this metal.
Tungsten displays the following properties:
Positioned among the transition metals in the periodic table, tungsten exhibits multiple oxidation states: +2, +3, +4, +5, and +6. Its atomic number is 74, and with a relative atomic mass of 183.84, it stays solid at room temperature because of its stable isometric crystal framework, which X-ray analysis can discern.
Tungsten resists corrosion from most acids except nitric and hydrofluoric acids. It can also react with alkaline oxidizers like potassium nitrate or sodium hydroxide but tends to remain generally resistant. However, at high temperatures, tungsten can quickly react with oxygen to form trioxides.
Tungsten exists as a mixture of five stable isotopes: tungsten-180, tungsten-182, tungsten-183, tungsten-184, and tungsten-186. Their respective proportions are approximately 0.12%, 26.5%, 14.3%, 30.6%, and 28.4%.
Tungsten, in its pure form, is a shiny white metal quite easy to handle. However, the presence of minute quantities of oxygen and carbon can make it fragile under severe stress and pressure.
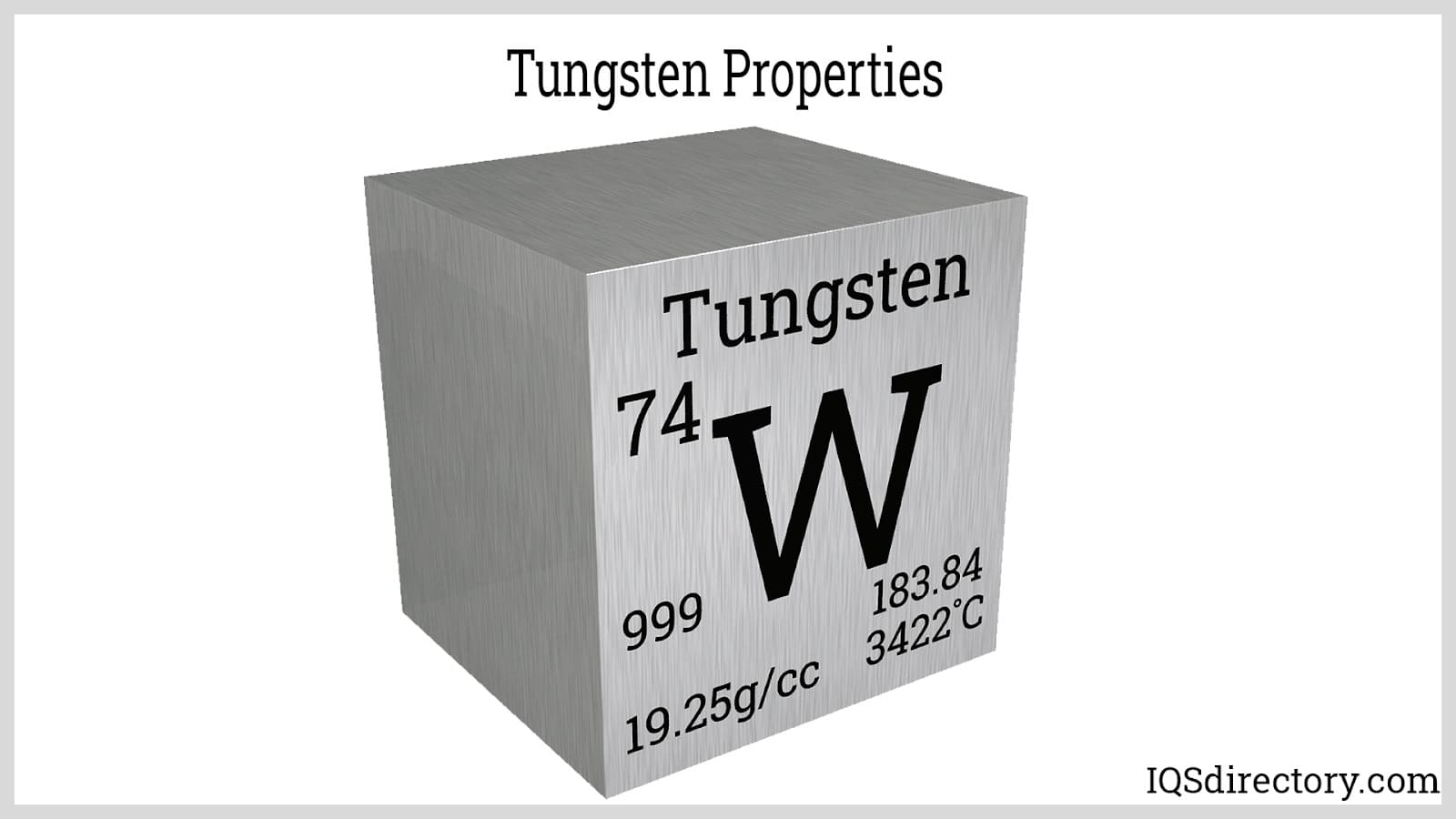
This metal is the densest engineering material, with a density of 19.25 g/cm³ due to its compact crystal arrangement. It boasts a melting point of 3,140°C and a boiling point of 5,700°C. It has the lowest vapor pressure among metals and achieves the highest modulus of elasticity at 400 GPa.
Tungsten has a minimal thermal expansion coefficient of 4.4×10⁻⁶ mm/°C, parallel to that of borosilicate glass, making it ideal for glass-metal seals. Moreover, tungsten is environmentally robust and does not easily break down.
As a metal, tungsten embodies numerous conventional metallic properties. It efficiently conducts electricity owing to its free electrons and remains an excellent conductor of heat. Tungsten alloys maintain these essential conductive properties, making them invaluable for electrical industries.
Tungsten is known for its corrosion resistance, distinguishing it as a unique metal. Despite its advantageous properties, tungsten can be brittle in its pure form. Therefore, it is generally alloyed with other metals to produce more robust and manageable compounds.
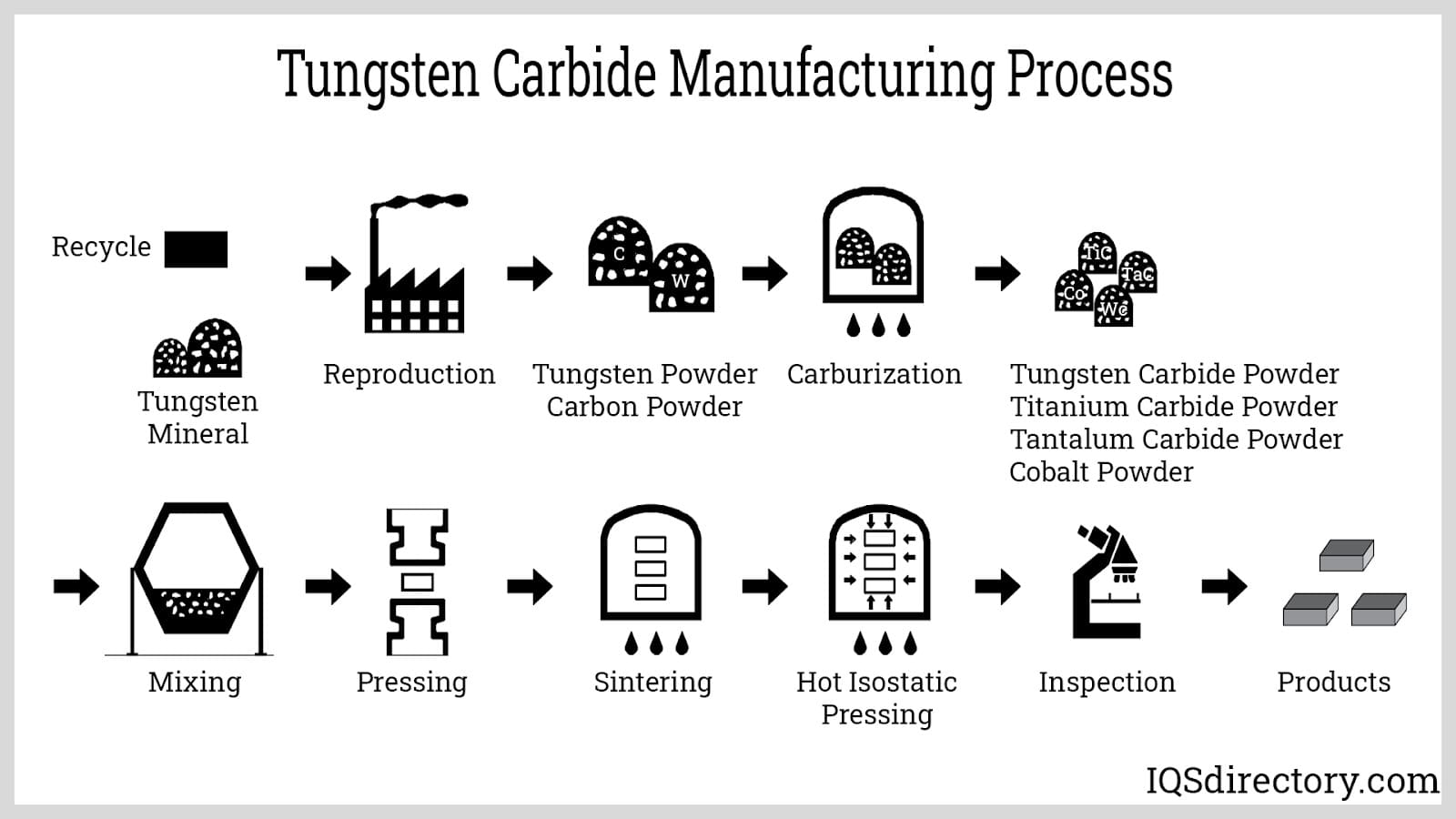
Tungsten goes through a multi-step extraction and refining process. Initially, tungsten ore is transformed into tungsten (VI) oxide (WO3). This oxide is then subjected to heating with hydrogen or carbon to create tungsten powder. Due to its high melting point, forming tungsten ingots is technically challenging and commercially unfeasible.
Tungsten powder is combined with small amounts of nickel or other metals before undergoing sintering, allowing nickel to diffuse and form tungsten alloys.
The powder can also be obtained by reducing tungsten hexafluoride (WF6) using hydrogen.
WF6 + 3H2 → W + 6HF
For manufacturing tungsten products, sintered tungsten alloys are molded into billets, later processed into foils, bars, plates, or sheets by grounding, drawing, die cutting, or molding. Some articles may need additional processing steps.
Tungsten products come in three primary forms: black (with a lubricant layer), oxide, and cleaned (surface stripped with chemicals). The ground form is further refined using diamond or silicon carbide tools for precision and smoothness.
Items made from tungsten are adaptable and find usage in manufacturing tungsten electrodes, drilling equipment, light bulbs, industrial machines, tools, military armament, material coatings (such as for boats), X-ray screens, and construction tools.

Tungsten alloys are instrumental in weapon production and developing effective heat sinks for thermal management. They are also essential in turbine blade fabrication and heavy-duty equipment manufacturing, such as weights and ballasts used in fitness applications.
The corrosion resistance of tungsten extends tool lifespan and quality. Tungsten is used for producing electrodes suitable for high-temperature applications, capable of withstanding water without rust or degradation.
Tungsten is integral to welding equipment for transmitting high voltage and currents across metal sheets. Its applications also include electrical discharge machines and tungsten gas arc welding setups.
Tungsten has five primary alloys: alloyed tungsten, cemented carbide, tungsten carbide, tungsten-based chemicals, and pure tungsten. These alloys are classified based on their nominal tungsten content and the ultimate tensile strength of the alloy.
Tungsten carbide is an alloy made from tungsten and carbon in equal proportions. It starts as a fine gray powder that is processed into various forms and materials. Known for its exceptional strength, tungsten carbide can withstand exposure to acids, alkalis, oxygen, and water. It is twice as strong as high-grade steel and denser than both titanium and steel. There are more than 20 grades of tungsten carbide powder, differing in grain size, tensile strength, hardness, and melting points.
The production of tungsten carbide involves sintering and pressing the powder into robust and durable products, tools, and components. Its exceptional strength makes tungsten carbide highly valuable in industries such as mining, construction, and metalworking. In fact, approximately 60% of all tungsten carbide alloys are specifically produced for these applications.
Tungsten carbide is widely used in tool manufacturing where it is simply referred to as carbide and used to produce the cutting edges of tools like saws and drill bits. In CNC machining, tungsten carbide is an essential part of the process for shaping and forming programmed parts and components. Unlike hand tools, tungsten carbide tools for CNC machining have the complete tool made of tungsten carbide due to the rigors and stress that CNC machining places on its tools. Its use gives CNC machining tools a longer useful life.
This alloy boasts exceptional strength and is highly resistant to wear from chemicals such as acids and alkalis, as well as the gradual impacts of oxygen and water. It is twice as strong as high-grade steel and denser than both titanium and steel. Tungsten carbide powder comes in up to 20 different grades, each varying in grain size, tensile strength, hardness, and melting points to suit a range of applications.
Tungsten is processed through sintering and pressing to create a variety of robust and long-lasting products, tools, and components. Its remarkable strength makes tungsten carbide particularly valuable in sectors such as mining, construction, and metalworking. In fact, around 60% of all tungsten carbide alloys are specifically produced for these industries.
Tungsten carbide alloy is produced by reacting tungsten with carbon at extremely high temperatures, typically between 1,400 and 2,000°C. Alternatively, a low-temperature fluid bed method can be used, where tungsten metal or blue tungsten oxide (WO3) is combined with a carbon dioxide or carbon monoxide and hydrogen mixture at temperatures ranging from 900 to 1,200°C.
Additional chemical vapor techniques have been developed, including the following:
Tungsten hexachloride is reacted with hydrogen as a reducing agent and methane as the source of carbon at a steady temperature of 670°C.
WCl6 + H2 + CH4 → WC + 6HCl
Tungsten hexafluoride is also reacted with hydrogen as a reducing agent and methanol as a source of carbon at a temperature of 350°C.
WF6 + 2H2 + CH3OH → WC + 6HF + H2O
Tungsten carbide features a macrocrystalline structure, which contributes to its superior strength and durability compared to other tungsten alloys. The presence of carbon lowers the melting point of the alloy. While tungsten carbide exhibits high resistance to acids, it can react with certain elements when exposed to high temperatures.
Tungsten carbide is both hard and durable, scoring 9 on the Mohs scale of mineral hardness. It boasts excellent tensile and compression strength. Although it does conduct electricity, its conductivity is lower compared to other materials.
Tungsten carbide is utilized in making cutting and drilling tools due to its high abrasion resistance and ability to withstand elevated temperatures. This alloy produces exceptional cutting tools that retain their sharpness longer than steel tools, making them highly preferred in various applications.

Another use of tungsten carbide is in ammunition production. It is employed in making armor-piercing rounds due to its extreme toughness. This alloy is utilized to create high-grade ammunition capable of penetrating even the toughest surfaces, such as tanks and fortified bunkers.
Another prevalent application of tungsten carbide is in mining and drilling through tough materials. It is commonly used to produce rock drill bits, roller cutters, plow chisels, and tunnel boring equipment.
The alloy also serves as an efficient neutron reflector in the nuclear industry. Additionally, carbide tips are utilized in sporting equipment, such as racing shoe studs and trekking poles. Tungsten carbide's excellent corrosion resistance makes it a popular choice for surgical instruments and certain types of jewelry.
Inhaling tungsten carbide dust poses significant health risks, including respiratory conditions such as fibrosis.
This is a widely used tungsten alloy, specifically tungsten carbide combined with cobalt as a binder, creating a cemented structure. The addition of cobalt enhances the toughness of tungsten, reducing its brittleness under high pressure and making it suitable for demanding structural applications.
Cemented carbides generally consist of a metallic matrix with carbide particles serving as the aggregate and the metal binder forming the matrix. This structure is similar to that of a grinding wheel, where fine abrasive particles are embedded in a binding matrix.
Hot isostatic pressing involves merging carbide particles with a binder, which is in a liquid form, while the carbide grains stay solid due to their significantly higher melting point. This process results in a composite material with unique properties in its final form.
The thermal expansion of cemented carbide depends on the cobalt content used as a metal binder. Generally, as the percentage of cobalt increases, so does the thermal expansion of the material.
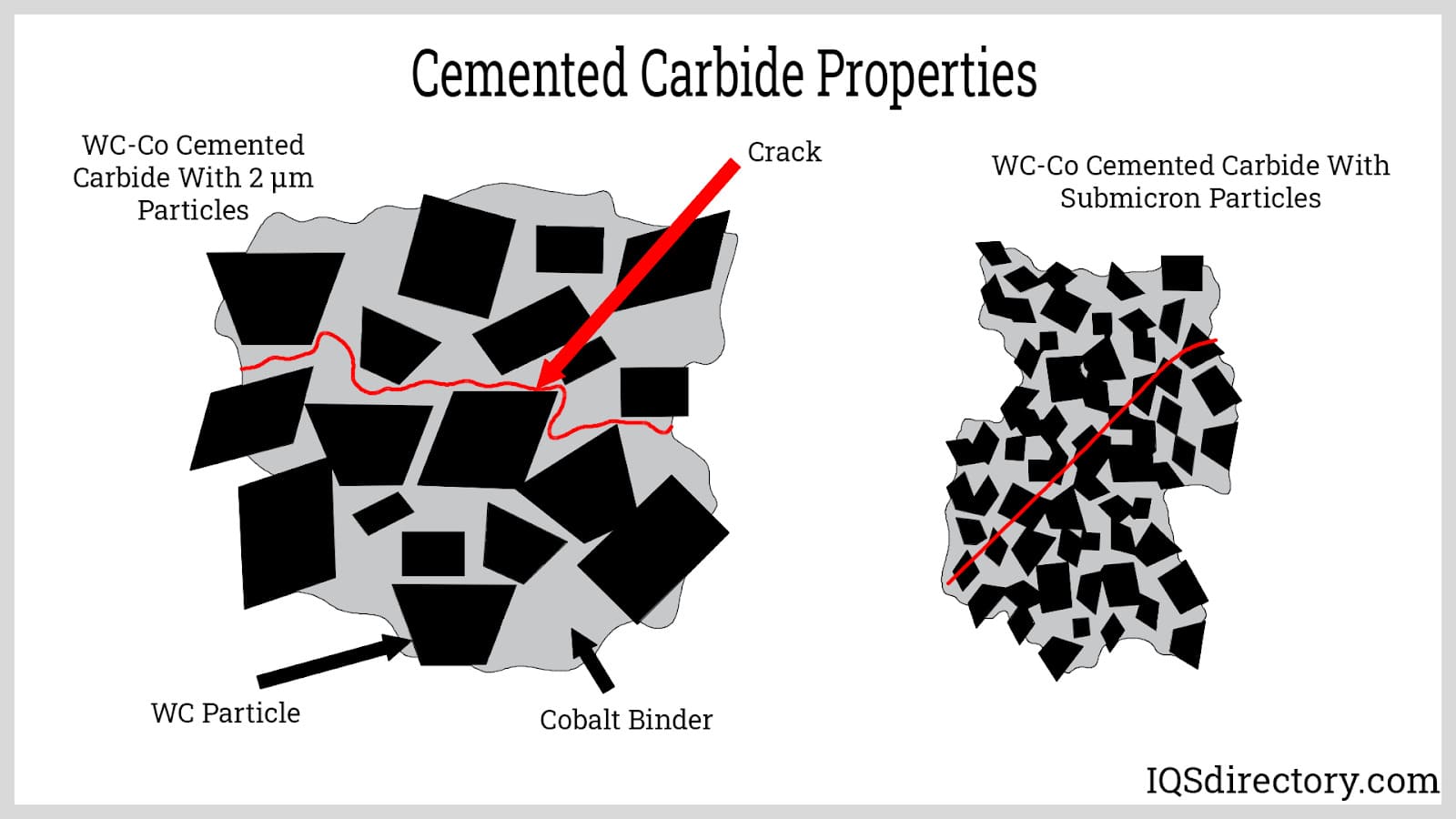
Cemented carbide is commonly used for inserts in metal cutting tools. Its reinforced structure reduces susceptibility to vibrations, making it crucial for applications in boring and threading equipment.
Cutting tools naturally wear out over time, so they are often coated to prolong their lifespan. Common coatings include titanium aluminum nitride, titanium nitride, titanium carbide nitride, titanium carbide, and aluminum titanium nitride. Tungsten alloys are also used for coating to enhance the durability of these tools.
Mining and tunneling equipment often feature cemented carbide tips, commonly referred to as bottom bits, making these tools highly effective for drilling and excavation worldwide. Cemented carbide is also used in the production of hot and cold rolls for milling operations. Additionally, this alloy is employed in industrial pump pistons for high-performance applications, such as those found in nuclear facilities.
Alloyed tungsten refers to a range of alloys created by combining tungsten with various metals, such as copper and iron. These alloys are valuable for both industrial and commercial uses. For example, tungsten-nickel-iron alloys benefit from nickel's contribution to increased density, strength, and ductility. Another notable alloy is tungsten-nickel-copper, which features copper to make the alloy non-magnetic. This non-magnetic property is crucial for applications in oncology systems and tasks where shielding of electrical sensors is essential.

Alloys of tungsten that include copper are notably non-magnetic. These alloys typically have a low tensile strength, ranging from 500 to 700 MPa, and reduced ductility. Despite these characteristics, their considerable strength makes them suitable for use as conductors in the production of heating coils.
Tungsten-copper alloys are primarily utilized in the production of heating coils due to their excellent resistance to high temperatures. This alloy is commonly found in water heaters, kettles, ovens, and certain stove plates.

Heavy metal tungsten alloys have a high tungsten content, often up to 90%, with only a small proportion of additional metals. For instance, adding around 2% thorium oxide improves thermionic electron emission, making it a typical example of such an alloy.

In its pure state, tungsten is an excellent electrical conductor and is primarily employed in electrical applications. In electronics, pure tungsten serves as a connecting medium for components on circuit boards.
Tungsten is also utilized in the production of pigment phosphors, organic dyes, and X-ray screens.
Tungstic acid is a yellow, fine-grained powder with very small particle sizes. It is highly reactive and exhibits exceptional chemical purity. Its primary application is as a sintering enhancer in ultra-high-temperature ceramics.
This chemical, known as sodium tungstate dihydrate, appears as a white crystalline powder with high chemical purity. It dissolves completely in water without leaving any residues.
Ammonium metatungstate is a tungsten-based chemical that appears as a white crystalline powder. It dissolves completely in water without leaving any residues.
Tungsten trioxide is a powder that can be yellow or green, noted for its high purity and ultra-fine crystal structure.
This tungsten carbide chemical is a black, fine-grained powder. It is commonly used to create a suspension that enhances the density of SPT-3.
Tungsten was first identified in 1783 through the charcoal reduction of the oxide extracted from wolframite. It has been utilized for centuries, notably in the production of porcelain for pottery. In various countries, tungsten is known as wolfram, a term derived from the German words "wolf rahm," meaning "wolf's foam." The name tungsten originates from Swedish, meaning "heavy stone."
Tungsten serves as a base and alloying element, with tungsten carbide being one of its most common alloys. Key tungsten minerals include scheelite and wolframite. Tungsten's first major modern application was as a filament material in light bulbs, a use that remains widespread today.
Tungsten carbide is a compound composed of equal amounts of tungsten and carbide atoms. It appears as a gray powder that is compressed and shaped through sintering for various applications, including equipment, cutting tools, abrasives, armor-piercing shells, and jewelry. Like tungsten, tungsten carbide has a high melting point of 5,200°F (2,870°C) and a boiling point of 10,830°F (6,000°C).
There are two main types of tungsten carbide compounds: tungsten carbide (WC) and tungsten semicarbazide (W2C). Additionally, tungsten carbide is categorized into two forms: α-WC, which has a hexagonal structure, and β-WC, which has a cubic structure that is stable at high temperatures.
Throughout the centuries, tungsten's popularity has increased due to its exceptional resistance to high temperatures, low vapor pressure, high melting point, stiffness, conductivity, and density. It is often alloyed with metals such as nickel, copper, and iron to create a more malleable microstructure that improves machinability and ductility while preserving tungsten's superior properties. Adding tungsten to any metal enhances the alloy's quality, leveraging tungsten's strength and rigidity.
Tungsten is the second hardest material on Earth but is naturally brittle, making it challenging to work with. While pure crystalline tungsten is more ductile and easier to cut, it is costly and typically reserved for specialized applications. To enhance its ductility and workability, tungsten is usually heated during processing.
The powder form of tungsten is the raw material for processing tungsten products and alloys. It is made into wire, rods, tubes, plates, and various shapes. Tungsten powder is mixed with other metal powders, such as molybdenum, rhenium, copper, and high density tungsten. When it is produced as tungsten carbide powder, it is used to produce hard alloy tools. Although tungsten powder has many uses, it has to be handled with care since it can spontaneously ignite in the open air.
Tungsten carbide is produced by combining tungsten powder with carbon powder through various methods. One approach involves heating the powders to temperatures above 2,200°C to initiate a chemical reaction. This high-temperature carburization results in a stoichiometric ratio of one tungsten atom to one carbon atom, forming crystals that are 100 µm or larger.
The resulting WC alloy from the combination process is more rigid than steel and boasts superior compression strength. Tungsten carbide (WC) is a highly stable alloy that resists deformation under extreme temperatures, whether hot or cold. In its monocarbide form, WC's hardness is comparable to that of diamonds and it offers excellent resistance to galling, abrasion, and impact. Its ability to withstand thermal shock makes it up to 100 times more durable than steel in extreme conditions.
When tungsten carbide is combined with a binder like nickel or cobalt, it creates a "cermet," or ceramic metal. This composite material is highly durable, capable of enduring cutting, oxidation, high temperatures, and severe wear, with a density of 15.7 g/cm³. Its exceptional toughness makes it challenging to machine, typically requiring diamond-coated tools, as most conventional bits are made from tungsten carbide itself.
The elastic modulus, or Young's modulus, measures a metal's stiffness or rigidity. For comparison, magnesium has an elastic modulus of 45 gigapascals (GPa), while tungsten has an elastic modulus of 407 GPa. When tungsten is combined with carbon to form tungsten carbide, the alloy achieves an elastic modulus of 600 GPa, making it nearly as hard as diamonds, which have an elastic modulus of 1,000 GPa.
The shear modulus, also known as the modulus of rigidity, measures a material's resistance to shearing or torsion forces, defined as the ratio of shear stress to shear strain. Tungsten has a shear modulus of 161 GPa, which is twice that of steel, which has a shear modulus of 80 GPa. Tungsten carbide, however, exhibits an even higher shear modulus of 274 GPa, surpassing both steel and tungsten.
Due to their high rigidity and stiffness, tungsten and tungsten carbide are brittle and not suited for high-tension applications. Brittle materials excel in compression. Tungsten carbide, for instance, has a compressive strength of 2,683 MPa at room temperature and maintains this strength despite temperature changes. In contrast, steel has lower compressive strength and its performance varies with temperature fluctuations.
In applications involving heat from friction or electricity, metals often experience changes in thermal conductivity due to temperature variations. Tungsten, however, maintains its thermal conductivity stability and is unaffected by temperature changes. This stability makes tungsten ideal for use in filaments, tubes, and heating coils. It retains its properties under thermal stress, making it suitable for a diverse array of applications.
Among the various properties of tungsten carbide, its hardness stands out as the most remarkable. High hardness indicates that a material is resistant to surface damage from scrapes, pitting, scratches, and abrasions. Hardness is typically measured using an indentation test, where a small sphere or indenter is pressed into the material's surface.
Among the various hardness scales, the Rockwell scale is the most commonly used. For measuring tungsten carbide, the Rockwell A scale is employed, specifically designed for very hard metals. In this test, a diamond indenter is pressed into the material. Tungsten carbide has a Rockwell A hardness rating of 94 HRA, which enables it to cut through hardened steel effectively.
Tungsten, like other metals, is available in various forms and shapes to facilitate shipping and meet customer needs. These forms include bars, sheets, and foil, which are produced from the extraction of tungsten ore. Tungsten also comes in different alloys, each tailored for specific applications and processes.
Tungsten bars are composed of 99.9% pure tungsten powder that has been shaped into various forms. These bars retain all of tungsten's properties, including its high density and resistance to high temperatures. When alloyed with other metals, tungsten bars can be produced in six different alloys and customized for specific applications.
As one of the rare metals found in the Earth's crust, tungsten has a density comparable to gold and is 1.7 times denser than lead. Working with pure tungsten bars can be challenging due to their extremely high melting point of 3,422°C (6,191.6°F). However, tungsten bars are known for their stability under various conditions, including exposure to water, air, and sulfuric acid. They do oxidize when exposed to the atmosphere.
Tungsten bars are produced through powder metallurgy. In this process, tungsten ore is first reduced to a fine powder and then chemically processed to create tungsten oxide. The tungsten is extracted from the oxide through heating, forming pure tungsten, which is then pressed into molds. After shaping, the bars are sintered to further consolidate and bind the metal powder.
Once processed, tungsten bars are sent to manufacturers for the production of various components such as cathodes, wire, pins, electrodes, stainless steel additives, and heating elements. Producers aim to supply tungsten in the appropriate form to meet the specific requirements of their customers.
Tungsten foil is a thin, smooth metal sheet with a black metallic sheen, produced by rolling tungsten sheets through cold or hot processes to achieve the desired thickness. The manufacturing of tungsten foil starts with tungsten bars that are rolled into flat sheets, which are then further processed to create the foil. The process includes vacuum annealing furnaces to strengthen the tungsten.
Stamped and drawn tungsten foil is utilized in high-temperature applications such as heat shielding, heating elements, and furnaces. It is also used in semiconductors due to its low thermal expansion coefficient and low electrical resistivity. Tungsten foil typically ranges in thickness from 0.05 mm (0.002 in) to 1 mm (0.04 in) and has a purity of 99.9%. It is available in widths from 100 mm to 400 mm (4 in to 16 in) and lengths from 400 mm to 1500 mm (16 in to 59 in).
Tungsten foil is silver gray with a metallic luster, a melting point of 3410o C (6170o F), and density of 19.35g/cm3. It is used to manufacture electric light source parts, medical shields, current collectors for aluminum ion batteries, heating elements, and heat shields. Additionally, tungsten foil is used to make tungsten evaporation boat.
Similar to tungsten sheets and foil, tungsten plates are flat pieces made from tungsten powder. The manufacturing process involves extracting tungsten from its ore, grinding it into pure tungsten powder, and then molding it into plates using heat and pressure. After shaping, the plates are annealed to improve their strength. While tungsten plates and sheets are similar, they are distinguished by their thicknesses.
Processing pure tungsten plates is challenging due to tungsten's exceptionally high melting point. Every step in the process must be carefully controlled to prevent damaging the plates. During the rolling process, manufacturers must precisely heat the rollers to match the specific rolling conditions.
Tungsten plates are available in a range of sizes and thicknesses, with most being cast. The standard thicknesses start at 6.35 mm (0.25 in), though custom thicknesses can be produced for specific applications. Tungsten plates are commonly used for shipping due to their manageable size and ease of handling.
Tungsten plates are utilized across various industries, including aerospace, construction, and electronics. They are used to manufacture turbine blades for generators and aircraft engine components because of their high melting point and exceptional strength. Additionally, tungsten plates are processed into radiation protection materials due to their density, radiation absorption properties, and resistance to corrosion.
Tungsten rods are cylindrical extrusions available in various lengths to suit different applications. They come in a range of diameters, shapes, sizes, and tungsten grades. Their widespread use is attributed to their remarkable strength, durability, and corrosion resistance. Tungsten rods can be machined, ground, and shaped to create a diverse range of parts and products.
Tungsten rods are available in various shapes such as round, square, rectangular, octagonal, hexagonal, and oval, depending on the manufacturer and intended use. However, a notable challenge with tungsten rods as raw materials is the difficulty in fabricating them into finished products due to tungsten's high melting point.
Tungsten rods have many uses and are a critical part of engineering designs and industrial manufacturing. A common use for tungsten rods is as cutting tools, which is superior to cutting tools made of aluminum or steel. In medicine, tungsten has been perfected to be used as implants and in medical procedures that require exposure to extreme temperatures due to sterilization. The factor that makes tungsten rods the first choice for manufacturing, production, and industrial applications is its exceptional wear resistance, which makes it perfect for operations that require repeated use of a tool.
Tungsten sheet is a flat form of tungsten that is thicker than foil but thinner than tungsten plate. It is produced from tungsten slabs refined during processing. Tungsten sheets are distinguished from foil and bars by their thickness, which ranges from 1.5 mm to 2.2 mm (0.06 in to 0.09 in). Tungsten material thicker than 0.25 mm (0.1 in) is categorized as tungsten plate.
The surface of tungsten sheets can vary, including finishes such as shiny, matte, satin, or rolled, depending on their thickness. The sheets are produced by isostatically pressing and sintering high-purity tungsten powder into ingots using powder metallurgy techniques. Before reaching the final finish, the ingots are deformed and heat-treated. The resulting blanks are then further processed by hot or cold rolling into sheets.
Tungsten sheets, like tungsten plates and foils, are prized for their radiation shielding properties and are used in manufacturing electronics, cables, and shipbuilding materials. They are particularly valuable for specialty applications that leverage their unique attributes. Tungsten sheets are often preferred over other high-density metals such as lead, tantalum, and uranium. Lead and uranium are avoided due to their toxicity, while tantalum, though a viable alternative, is very costly compared to tungsten.
This section will explore the uses and benefits of Tungsten metal.
Tungsten is commonly employed in making filaments for incandescent light bulbs. However, these bulbs are being gradually replaced in many places because they are not energy-efficient and convert more energy into heat than into light. When an electric current flows through the filament, it heats up and emits light. Tungsten’s ability to endure high temperatures allows the filament to remain functional and continue glowing despite the intense heat.

Tungsten is frequently used in the production of cutting tools. It can be fashioned into blades or combined with other elements such as nickel, copper, or carbon to enhance its properties. Tools made from tungsten or its alloys are exceptionally hard and can endure high temperatures, allowing them to operate for extended periods without melting or deteriorating.
Tungsten is also utilized in drilling and boring equipment. The metal’s exceptional hardness makes it ideal for drill bits and heavy-duty earth-boring machinery. Additionally, tungsten is used in dental drills. Cemented carbide, a tungsten-based material, is commonly employed in high-speed tools for mining refineries and other demanding applications. Due to its durability, tungsten is also popular in the creation of jewelry.
Calcium and magnesium tungstate are employed in fluorescent lighting applications. Tungsten is also utilized as a catalyst in the reduction of certain complex compounds. Additionally, tungsten plays a role in the production of magnetrons used in microwave ovens. Old television sets with cathode ray tubes incorporate tungsten due to its ability to endure the high temperatures generated over time.
Tungsten is employed in applications where high density is essential. Common uses include producing counterweights, ship anchors, and tail ballasts for engines in rockets, airplanes, and other vehicles.
Tungsten finds several important military uses. Its strength and hardness make it suitable for producing armor-piercing ammunition. Tungsten or its alloys are sometimes used in the manufacture of missiles, grenades, shells, and large rounds designed to penetrate heavily fortified targets such as tanks or bunkers.
Tungsten (IV) sulfide serves as a lubricant capable of enduring extremely high temperatures, making it ideal for use in high-speed tools.
Advantages of utilizing tungsten metal include:
Among all pure metals, tungsten boasts the highest melting point at 3,244°C. This exceptional property allows it to be utilized in environments with extremely high temperatures where other metals would fail.
Tungsten has a high density of 19 g/cm³, attributed to its microcrystalline structure. This characteristic makes it valuable in applications that need substantial mass within compact dimensions.
Among all pure metals, tungsten has the lowest coefficient of thermal expansion. Unlike steel, tungsten remains highly stable at elevated temperatures and does not change in form or size.
Due to its excellent conductivity and general inertness, tungsten is commonly employed in electrical devices that operate under high levels of radiation. It also serves as an ideal material for electrodes in the electrolysis of various substances.
Tungsten's exceptional resistance to corrosion allows it to be used in diverse applications where corrosion is a concern. It performs well in outdoor environments exposed to water and acids. Typical uses of tungsten include fishing lures, ship construction, and certain types of jewelry.
Despite its strength, tungsten can be drawn into extremely thin wires without breaking. For example, the filaments in light bulbs are made from tungsten, allowing them to endure high temperatures while remaining functional.
In its pure form, tungsten is quite brittle and can easily fracture. It cannot withstand stretching, compression, or twisting without losing its shape. This fragility is due to its rigid structure, which limits its ability to be manipulated without failure.
Inhaling tungsten can irritate the mucous membranes and the lungs. It is considered highly toxic, and uncontrolled exposure could pose significant risks to both human health and the environment.
Tungsten is a valuable metal with a high cost due to its unique properties. Its rarity and the growing demand contribute to its continued high price.
Tungsten is a dense metal, which makes it challenging to work with. For example, tungsten jewelry can be quite heavy, often causing discomfort for the wearer. Similarly, in the production of earth-boring equipment, the metal's weight results in high transportation costs due to the difficulty of moving such heavy machinery.
Although tungsten is expensive, it does not retain its value upon resale. Unlike gold, which can be fashioned into various jewelry pieces and resold at a value close to its original purchase price, tungsten does not hold the same resale value.
Compared to other metals, tungsten has the following specific applications and uses:
Tungsten and its alloys are used to manufacture durable tools for cutting and crushing. Unlike steel, which can wear out and break when used on tougher materials, tungsten offers superior longevity and strength, making it the ideal choice for heavy-duty applications.
While tungsten is a good conductor of electricity, its high cost, weight, and brittleness make it less favorable compared to other metals. Copper, aluminum, and steel are generally preferred for electrical conduction due to their more advantageous properties.
Although tungsten is a hard and tough metal, it is not ideal for use in the construction industry. Its brittleness makes it unsuitable for large structural applications. Instead, lightweight and strong materials such as reinforced steel are more appropriate for building projects.
ATI offers a wide range of metals, including various types of tungsten, known for their high performance and superior quality. In addition to standard metals, ATI manufactures specialty metals to fulfill specific customer requirements and industrial demands. With over 25 years of experience, the company processes tungsten in the Dallas, Texas area.
Betek specializes in manufacturing tungsten carbide tools, systems, and wear protection. The company produces high-quality tools tailored to specific industrial applications, ensuring a longer lifespan and reducing the need for frequent tool replacements. While focusing on current needs, Betek is also committed to future innovations to address evolving demands and requirements.
Buffalo Tungsten produces tungsten and tungsten carbide powders of the highest quality. They offer a range of powders including fine, coarse, crystalline, ultra-high purity, high density, low grade, and granulated tungsten. Their powders boast a purity of 99% with particle sizes ranging from 10 microns (µ) to 50 µ. The company also provides ultra-high purity 5N tungsten metal powder, with a purity level of 99.999%. Buffalo Tungsten enhances the density, particle size, and flow characteristics of their powders through additional processing.
Starck Solutions manufactures high-quality tungsten chemicals, metal, and tungsten carbide. Their product range includes various tungsten forms, from tungstate to tungsten carbide, available in both ultrafine and coarse grain sizes. The company is committed to adhering to the highest standards in tungsten production and works closely with customers to develop innovative, customized solutions that meet and exceed their requirements.
Federal Carbide produces a variety of tungsten products, including tungsten heavy alloy, tungsten carbide, tungsten carbide seal rings, and tungsten heavy metal alloy radiation shields. Their tungsten heavy alloy items are available as machinable blanks or custom parts tailored to specific customer requirements. Their product range spans the full spectrum of the tungsten market, including components for engines, helicopter rotors, firearms, and golf club weights. Federal Carbide emphasizes providing the hardest man-made metal, tungsten carbide, at the lowest cost with fast and efficient delivery. The company's commitment to hard work and quick responses to customer needs underpins its goals.
In summary, tungsten is a naturally occurring metal found on earth. It is a very unique metal as it has a very substantial chemical structure. It has a very high melting and boiling point and is a very dense metal with an over the top weight and density. Despite all these excellent characteristics, tungsten is a very brittle metal and cannot be used as would other metals like copper and iron.

Aluminized steels are steels that have been hot-dip coated with pure aluminum or aluminum-silicon alloys. This hot-dip coating process is termed hot-dip aluminizing (HAD)...
Aluminum 1100 is the softest of the aluminum alloys, which makes it easy to shape and form into a wide range of products for industrial and home use. It can be cold and hot worked but is frequently shaped by...

The term "aluminum coil" describes aluminum that has been flattened into sheets where their width is significantly higher than their thickness and then "coiled" into a roll. Stacks of individual aluminum sheets are difficult to...
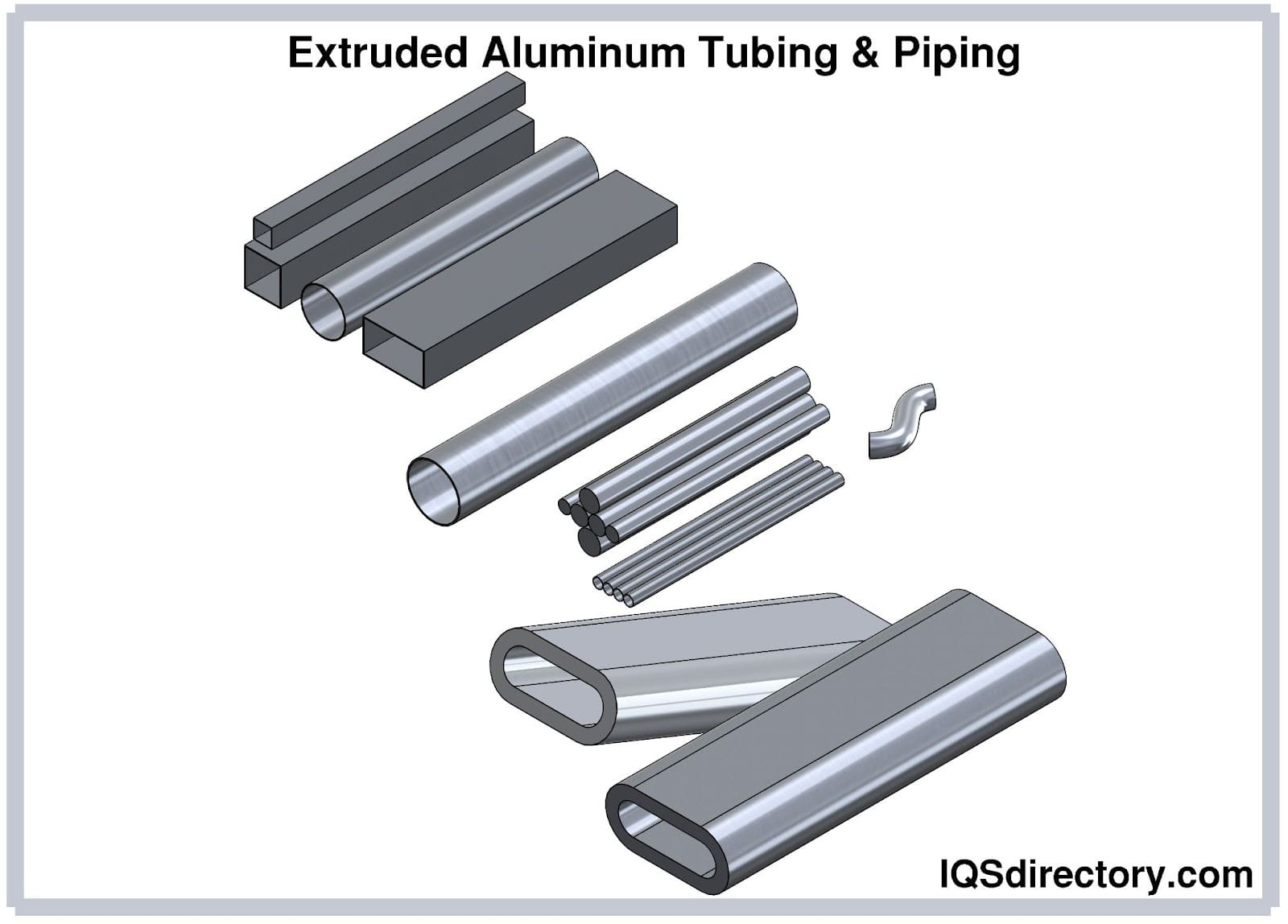
Aluminum piping and tubing is silvery-white, soft, and ductile. The metal belongs to the boron group. Aluminum is the third most abundant element present on earth. Aluminum has low density. When exposed...
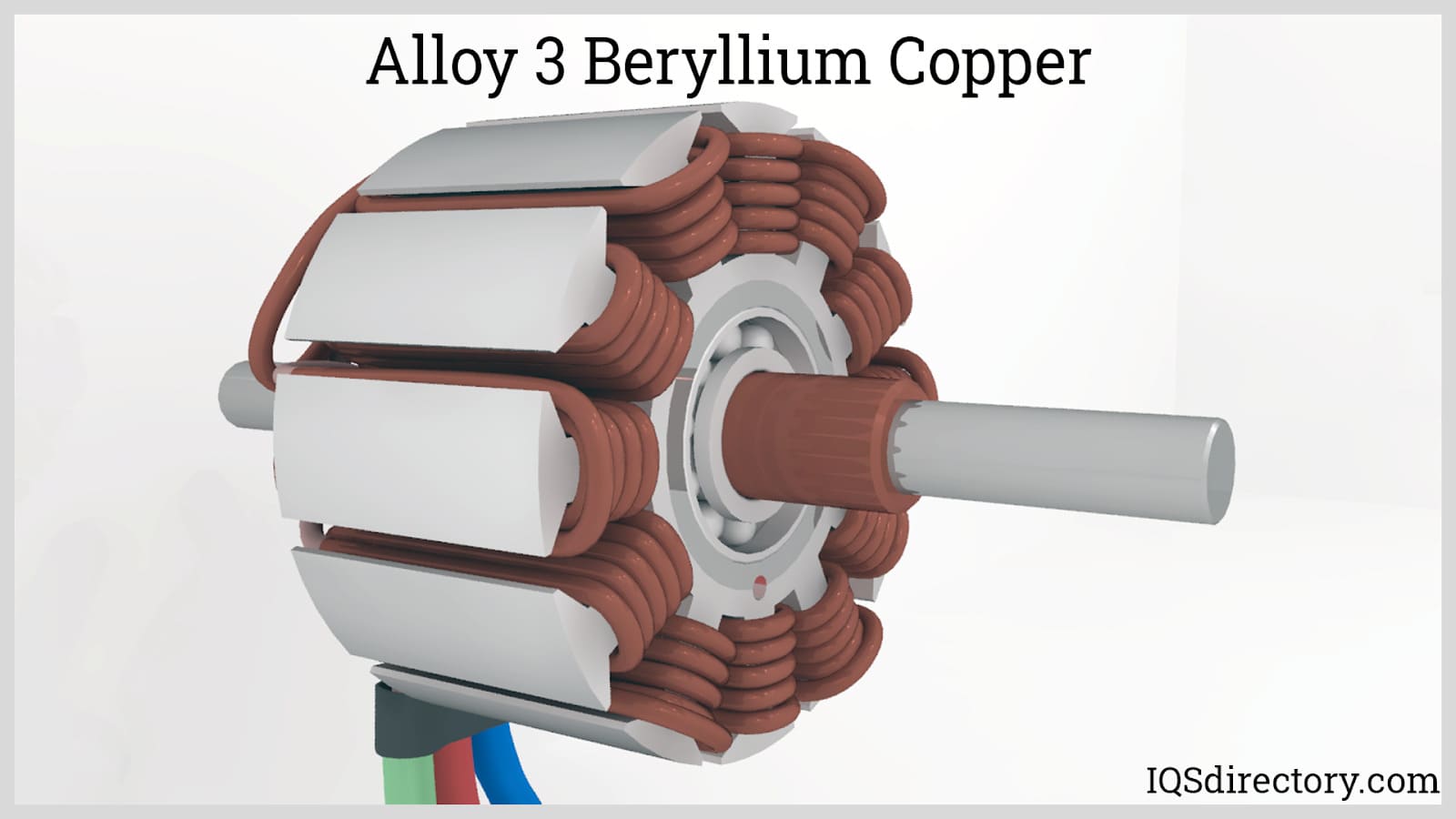
Beryllium Copper is a versatile copper alloy that is valued for its high strength and hardness, combined with good electrical and thermal conductivity. It is a non-ferrous, non-magnetic, and non-sparking metal alloy...

A variety of copper-zinc alloys are referred to together as brass. Different ratios of brass and zinc can be used to create alloys, which produce materials with various mechanical, corrosion, and thermal properties...
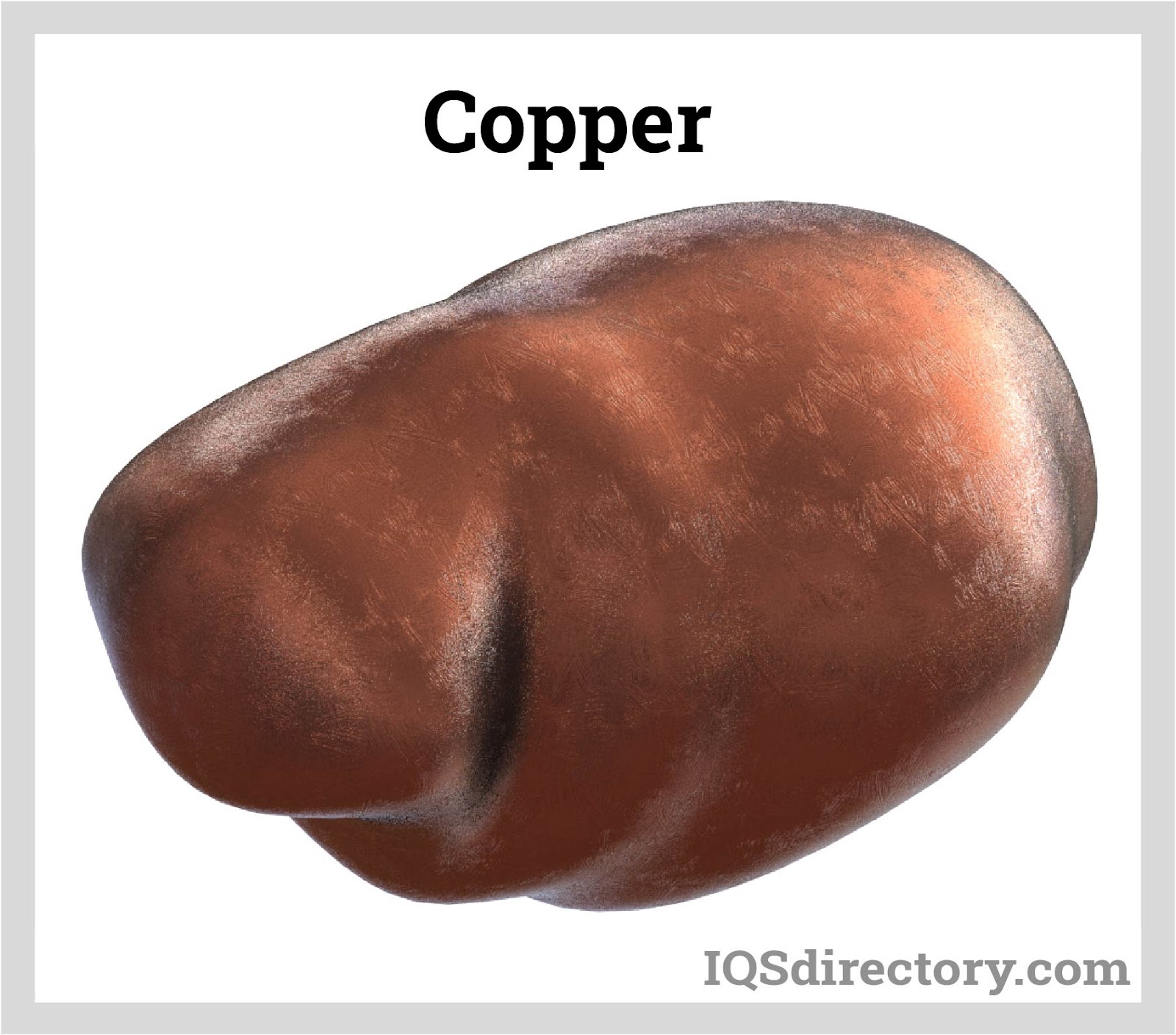
Copper is a ductile, malleable, and reddish-gold metal with the capacity to effectively conduct heat and electricity. Brass and bronze, two commonly used alloys, are created when copper is combined with...

The copper sheet is a highly malleable and workable metal with outstanding electrical and thermal conductivity and corrosion resistance. Copper (Cu) is a reddish, very ductile metal that belongs to Group 11 of the periodic table...

Metals are a group of substances that are malleable, ductile, and have high heat and electrical conductivity. They can be grouped into five categories with nickel falling in the category known as transition metals...

Stainless steel grade 304 is an austenite stainless steel that is the most widely used and versatile of the various grades of stainless steel. It is a part of the T300 series stainless steels with...

Stainless steel is a type of steel alloy containing a minimum of 10.5% chromium. Chromium imparts corrosion resistance to the metal. Corrosion resistance is achieved by creating a thin film of metal...

Stainless steel grades each consist of carbon, iron, 10.5%-30% chromium, nickel, molybdenum, and other alloying elements. It is a popular metal used in various products, tools, equipment, and structures that serve in many industrial, commercial, and domestic applications...

Steel service centers are companies that specialize in procuring steel directly from mills and manufacturers and supplying them to the customers. They are fundamental to the steel supply chain...

Stainless steel can be fabricated using any of the traditional forming and shaping methods. Austenitic stainless steel can be rolled, spun, deep drawn, cold forged, hot forged, or stippled using force and stress...

Stainless steel tubing is a multifaceted product that is commonly utilized in structural applications. Stainless steel tubing diameters and variations vary greatly based on the application requirements and are...

Titanium metal, with the symbol Ti, is the ninth most abundant element in the earth‘s crust. It does not occur in large deposits, yet small amounts of titanium are found in almost every rock...
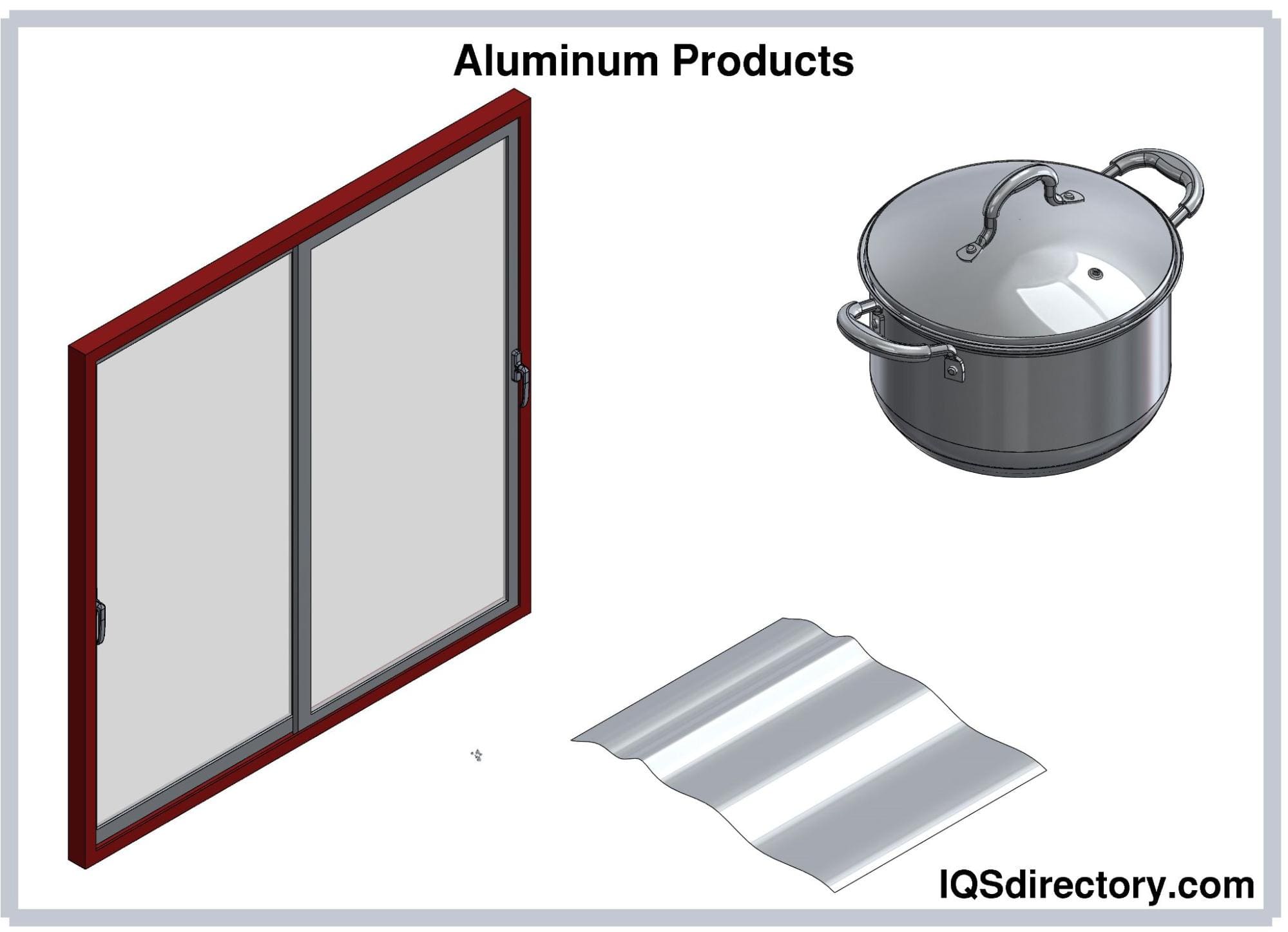
Aluminum is the most abundant metal on the Earth’s crust, but it rarely exists as an elemental form. Aluminum and its alloys are valued because of their low density and high strength-to-weight ratio, durability, and corrosion resistance...