Nickel
Nickel (Ni) is a naturally occurring metallic chemical element. Its atomic number on the periodic table is 28 and its atomic weight is 58.71. Nickel is essential for healthy plant life. For that reason, it is found not only in rock, but also in most fruits, vegetables, nuts and the food products derived from them, like wine and chocolate.
Because it is naturally occurring, it must be mined from deep within the earth instead of synthetically created in a lab. Called nickel ore, there are two main types of ore deposits: laterites, which are mainly composed of nickeliferous limonite and garnierite, and magmatic sulfide deposits, which are primarily composed of the ore mineral pentlandite.
In general, nickel has a silvery-white color, high toughness, is ferromagnetic and has excellent resistance to corrosion and rust. Some of its additional beneficial properties include its malleability, ductility, alloy-ability and high heat resistance—it has a melting point of 1453 degrees Celsius.
Quick links to Nickel Information
The History of Nickel
Nickel has been used for thousands of years; we have traced back the use of nickel metal back to 3500 BC in Syria, when some of their bronzes contained at least 2% nickel. Other ancient accounts of nickel are found in Chinese manuscripts written between 1700 and 1400 BC, which describe "white copper" (cupronickel). It is possible that China was the source of cupronickel used in the 2nd century BC to mint coins for the Bactrian kings Agathocles, Euthydemus II and Pantaleon. Because nickel ore is easily mistaken for silver ore, and it was not officially identified and isolated as an element until 1751 by Swedish chemist Axel Cronstedt. Before then, those who used nickel were doing so unwittingly.
Though nickel wasn’t officially discovered until the late 18th century, it got its name back in the 15th century. Its name comes from the Saxon term "Kupfernickel," which means "Devil’s copper." It earned this off-putting name from miners, who thought the metal, which is reddish-brown like copper, was 1) too hard to mine and 2) poisonous. Yes, the miners were being poisoned! However, it was not the nickel that was doing it, but arsenic.
In the 19th century, the newly identified nickel rose to prominence in the form of plating and alloys like "nickel silver," which is an alloy of nickel, copper and zinc. It does not contain silver. Nickel then quickly gained popularity as coin material. The first coins with copper alloyed silver minted in the USA began circulating in 1857. In 1881, the Swiss government began circulating coins made from pure nickel.
Early the following century, scientists discovered stainless steels. Quickly, they found the alloy greatly benefited from the addition of nickel. This is because nickel adds high temperature resistance and corrosion resistance. To this day, many grades of stainless steel contain nickel.
Today, nickel alloys are popular for those very same reasons, and nickel alloys can be found in settings as critical as chemical plants and jet engines.
Benefits of Nickel
Nickel is a great metal for many reasons. First, as compared to other metal materials, nickel offers more toughness, better corrosion resistance, a wider range of temperature resistance, and a variety of special electronic and magnetic properties. Second, nickel products have long working lives, with averages being between 25 and 35 years. In addition, nickel production is relatively low cost and energy efficient. Another aspect of nickel’s sustainability is its recyclability. Nickel and nickel alloys are one of the most heavily recycled materials in the world. In fact, it is estimated that half of the nickel content of any given stainless steel product today comes from recycled materials.
Nickel also has far reaching socio-economic benefits. For example, many important elements of human life are dependent on the performance of nickel-containing products. Such elements include: clean drinking water, food, power and heat, medicines and electronics. Often, nickel is the only material that can play its role. In addition, nickel is known as an "enabling technology" that helps users create new industries, products, jobs and benefits, and deliver improved performances in a wide range of sectors. For instance, nickel production provides jobs from work in the plant to maintaining public nickel-based products.
Production Process of Nickel
- Extraction Process
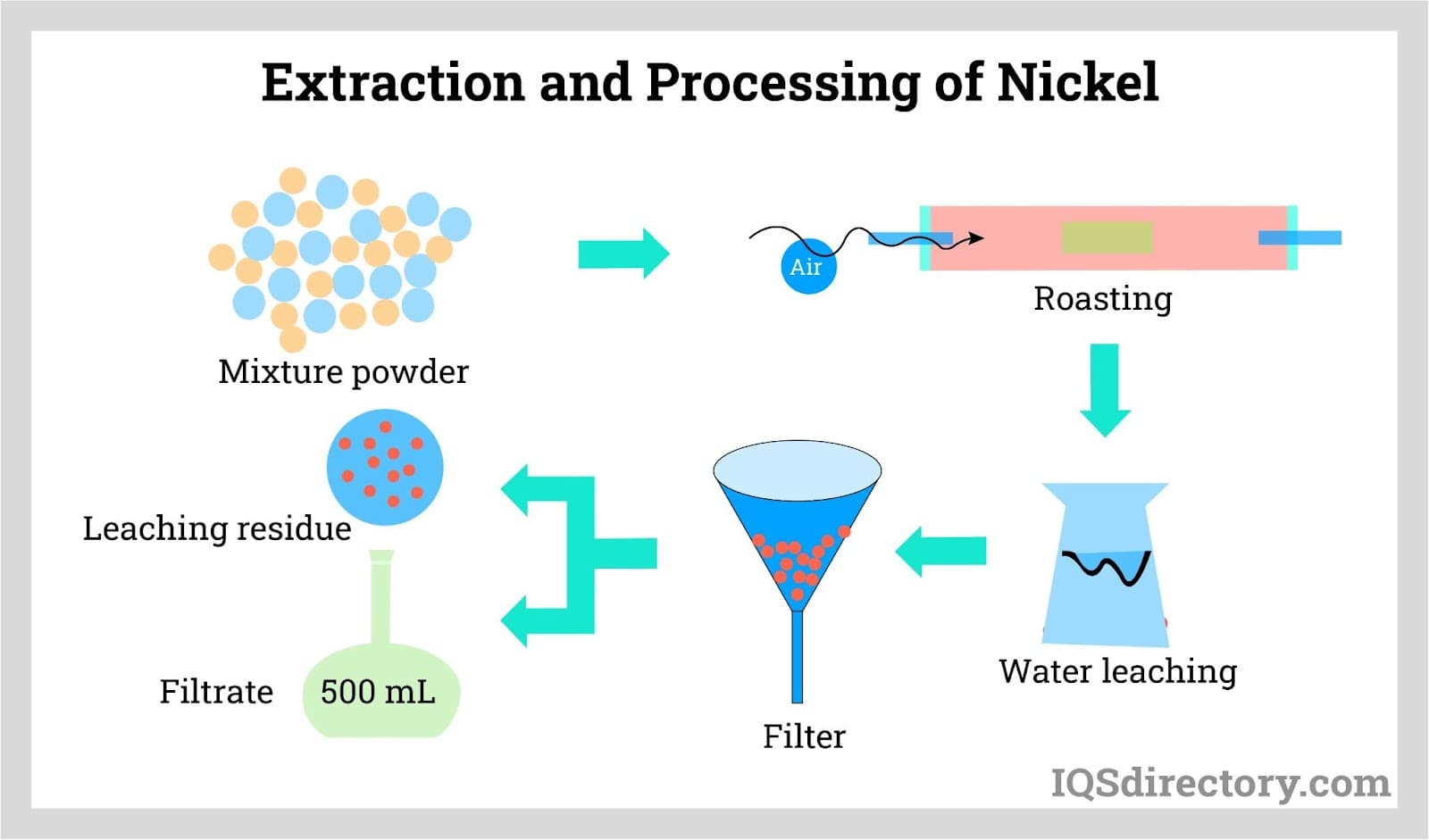
- Nickel ore is extracted from deposits through the process of extractive metallurgy, in which the raw metal material is extracted from the ore and purified into a refined form. The most common way the raw material is extracted is through pyrometallurgical extraction, but recent developments have made hydrometallurgy another viable process.
- Nickel Processing and Refinement
- Using conventional roasting and reduction processes, the refined form of nickel is able to yield a purity of greater than 75%. However, this level of purity can be increased through further processing. The Mond process, in which nickel oxides (nickel combined with oxygen) are converted into purified nickel, is able to achieve a purity of 99%. The nickel produced during this process is known as carbonyl nickel because carbon monoxide is utilized in the process.
- Shaping Process
- After nickel has been refined, nickel suppliers shape the metal into various shapes and parts for fabricators to use down the line. Nickel suppliers like to have in stock nickel shapes such as: nickel sheet, nickel plate, nickel rods, nickel bar and, sometimes, nickel tubing.
- To create these shapes, nickel suppliers use a wide variety of fabrication processes, such as extrusion, cold drawing or electroplating.
- Nickel Extrusion
- During nickel extrusion, nickel is heated to a molten state and then shaped by forcing the pliable nickel through a die. Nickel extrusion can produce nickel sheets, bars, rods and tubing. Note: Nickel extrusion is the least common form of nickel forming.
- Cold Drawing
- A more common process of shaping nickel is cold drawing. This is used to make tubes and wire. In cold drawing, no heat is added to the process, but the nickel tube or wire is drawn through a series of smaller and smaller dies, in order to reduce its diameter.
- Electroless Nickel Plating
- The most common way to form nickel is through electroless nickel plating. During electroless nickel plating, a catalytic reduction process of nickel ions occurs in an aqueous solution that contains a chemical reducing agent. As a result, nickel metal is deposited without requiring electrical energy. The main cause of the deposition of nickel ions as well as their reduction is the chemical reducing agent within the solution. In addition, the nickel ion deposits are very uniform in thickness in terms of both shape and size because the driving potential of the chemical reducing agent is constant at all points of the surface of the component, as long as the agitation is enough to ensure a uniform concentration of nickel ions and the reducing agent.
- Alloys and Compounds Made
- One of the transition elements, nickel is often combined with other elements in order to form various alloys and compounds. Typical alloying materials include: iron, chromium, copper, cobalt, aluminum and more. Iron-nickel (or nickel-iron) and nickel-chromium alloys are among the most popular, thanks to the strength that both iron and chromium lend. The most commonly formed nickel alloy is stainless steel, which is a nickel-chromium alloy composed of mostly iron and approximately 18% chromium and 8% nickel.
- Nickel is also used to make various other metal alloys including nickel 200 (which is made of 99.6% nickel), Invar® (a nickel-iron alloy), Monel®, Kovar®, Nichrome®, Inconel® and the various Hastelloys®. The three elements that are most commonly used on this list are Monel®, Kovar® and Inconel®.
- Monel®
- The general name for a series of nickel alloys that have a composition of nickel (approximately 67%), copper, iron and minute amounts of other elements. Although this nickel-copper alloy is very difficult to machine because it instantly hardens in reaction heat, it has incredible corrosion resistance. For this reason, it is often used in marine applications, such as trolling wire, pump shafts and seawater valves.
- Kovar®
- A term that encompasses a wide variety of nickel alloys that are compatible with the thermal expansion characteristics of borosilicate glass. Kovar® is mainly composed of nickel and cobalt, with minute amounts of silicon, manganese and copper, and is a ferrous alloy. Commonly used in electronics applications, Kovar® is often used as electroplated conductors for parts such as vacuum tubes and x-ray tubes.
- Inconel®
- Also referring to a family of nickel alloys, this nickel chromium alloy is actually a super alloy, meaning that it is a high-performance alloy. Exhibiting exceptional heat-resistance, Inconel® is composed mainly of nickel, chromium, molybdenum and niobium, with trace amounts of cobalt, manganese, copper, aluminum, titanium, silicon, carbon, sulfur, phosphorus and boron.
Nickel Images, Diagrams and Visual Concepts
 Nickel processed into a usable material.
Nickel processed into a usable material.
 Extractive metallurgy, a process used to extract the raw metal from its ore.
Extractive metallurgy, a process used to extract the raw metal from its ore.
 Inconel alloys contain nickel and chromium making it corrosion and oxidation resistant.
Inconel alloys contain nickel and chromium making it corrosion and oxidation resistant.
 Permalloy is a nickel alloy that contains 80% nickel and 20% iron, which makes the metal highly magnetic.
Permalloy is a nickel alloy that contains 80% nickel and 20% iron, which makes the metal highly magnetic.
 A nickel-based alloys this resistant to corrosive acid.
A nickel-based alloys this resistant to corrosive acid.
 This alloy contains iron, nickel, cobalt and trace amounts of manganese, silicon, and carbon, allowing it to have a low coefficient of thermal expansion.
This alloy contains iron, nickel, cobalt and trace amounts of manganese, silicon, and carbon, allowing it to have a low coefficient of thermal expansion.
 This alloy has a corrosion resistant, malleable, low coefficient of thermal expansion, and high strength.
This alloy has a corrosion resistant, malleable, low coefficient of thermal expansion, and high strength.
 The name for wrought nickel that has a purity of about 99.6%.
The name for wrought nickel that has a purity of about 99.6%.
Nickel Types
- Brushed Nickel
- A finish created by a rough surface going over the metal to create very small patterned lines, making a distinctive look, yet retaining its metallic luster.
- Casting Alloys
- Alloys used to form objects in molds, and they are more easily molded than other alloys.
- Cupro Nickel
- A nickel alloy made up of nickel, copper, iron and manganese (or other strengthening impurities). It does not corrode in seawater and is used in various marine applications.
- Inconel Alloy
- A nickel-base alloy, but also has chromium and iron, and it is used in gas turbine blades.
- Invar Alloy
- A trademark alloy of nickel and iron and is usually used in tuning forks, measuring tapes and other instruments.
- Kovar
- A registered trademark that refers to a low expansion alloy that is composed of iron, nickel, cobalt and trace amounts of the elements manganese, silicone and carbon.
- Monel
- A trademark alloy made up of mostly nickel, as well as copper, iron and other trace elements. It cannot be corroded by acids, and it can withstand fire in pure oxygen, but it is hard to machine because it hardens instantly.
- Nichrome
- A nickel-chromium alloy used for resistance heating elements because it can withstand high temperatures and has a high electrical resistance.
- Nickel 200
- Refers to wrought nickel that is commercially pure (99.6%).
- Nickel Alloys
- Alloys made up of more nickel than anything else.
- Nickel Bars and Rods
- Straight, solid products of nickel or nickel alloys that can be extruded. These products can have a variety of shapes, circular, triangular, square and more. Nickel rod refers to a solid, straight bar that can be either round or square, although round is much more common, and is composed of either high purity nickel or nickel-based alloys.
- Nickel Metal
- An element listed on the periodic table that is silver in color and both ductile and malleable.
- Nickel Plates
- Consist of rolled nickel and are used as a component in buildings and bridges.
- Nickel Pricing
- Usually only quoted for a short period—usually less than a week long—as the price of nickel alters regularly.
- Nickel Sheet
- A flat plane composed of nickel or nickel alloys.
- Nickel Suppliers
- Provide high purity nickel and nickel alloys, which share characteristics such as malleability, somewhat ferromagnetic, hardness, ductility, and electrical and heat-conductivity, to diverse companies for further processing.
- Nickel Tubing
- A hollow, cylindrical or rectangular rod that can be used as equipment components or to transport fluids or gases.
- Permalloy
- An 80/20 alloy of nickel and iron which is easily demagnetized and magnetized.
Nickel Applications
Nickel is valued for its positive properties, detailed in the section above. It is used to make products and both decorative and functional coatings. It is also used extensively to make alloys, which are in turn used to make products of all kinds.
Since nickel can be found in a wide range of metals, it is utilized in a correspondingly vast number of industries including: currency and coinage, consumer products, healthcare, chemical, industrial, food and beverage, electronics, military, transport, aerospace, architecture and marine.
Nickel Products Produced
Nickel and nickel alloys are used to make a wide variety of products. Approximately 65% of all nickel produced is used to manufacture stainless steel products, while another 20% is used to make other non-ferrous and steel alloys. Still another 9% is used to make plating, while about 6% is used to make other products, such as: coins (including the American nickel and the Euro), fittings and valves, electronic devices, electric guitar strings, microphone capsules, rechargeable batteries for portable equipment and hybrid cars, some light bulbs and microwave tubes, rocket motor cases, missile components, food processing equipment, pots and pans, kitchen sinks and medical equipment.
Nickel Grades
| Material |
Tensile Strength at Break (MPa) |
Tensile Strength, Yield (MPa) |
Modulus of Elasticity (ksi) |
| Pure Nickel |
45.0 - 317 |
59 |
30000 |
| All Nickel Alloys |
45.0 - 2070 |
59.0 - 4830 |
4060 - 34100 |
| Nitinol - High-Temperature Phase |
754 - 960 |
560 |
10900 |
| Nitinol - Low-Temperature Phase |
754 - 960 |
100 |
4060 |
| Inconel |
621 - 1550 |
195 - 1390 |
25700 - 32100 |
| Kovar |
517 |
345 |
20000 |
| Monel |
385 - 1100 |
134 - 790 |
~24500 |
| Nickel 200 |
379 |
103 |
10900 - 28000 |
*These figures are guidelines based on industry research; they should not be presumed accurate under all circumstances and are not a substitute for certified measurements. The information is not to be interpreted as absolute material properties nor does it constitute a representation or warranty for which we assume legal liability. User shall determine suitability of the material for the intended use and assumes all risk and liability whatsoever in connection therewith.
Overseas Nickel Market
Nickel ore deposits are dominated by Russia, producing 40% of the world's supply, Canada, producing 30%, and other countries, such as France, Cuba, Australia, New Caledonia and Indonesia, with smaller production percentages. The United States only has one active nickel mine, located in Michigan’s upper peninsula. Fortunately, nickel is heavily recycled, lessening some of the import burden and reducing prices. Also, between 2009 and 2012, the largest portion of our nickel imports came from Canada. With these relatively inexpensive and easy import statistics, there is little reason to look overseas for a supplier.
Things to Consider When Purchasing Nickel
It is easy to see why you’d want to purchase nickel material. Unfortunately, it’s less easy to know what type of nickel material. For the best results, we recommend you reach out to a supplier that will guide you through the market offerings. To that end, we’ve provided you with a list near the top of this page of capable and reliable nickel manufacturers and suppliers. Get a feel for who they are by browsing their respective webpages. To figure out which one is right for you, pick out three or four you find most promising, then reach out to them with your application details and requirements. Compare and contrast their answers. Pay attention not only to prices, but also secondary services, lead times, delivery policies and overall customer service. Decide which manufacturer is best for you, and then contact them to get started.
Nickel Terms
- Alloy
- A combination of two or more metallic elements that are usually dissolved into each other or fused together.
- Cold Forming
- Deformation of a metal at a low enough temperature to prevent re-crystallization during cooling.
- Conductivity
- A metal’s ability to conduct electricity. Nickel is a good conductor, and therefore is used in wires.
- Ductility
- The capability of a metal, such as nickel, to allow deformation or shaping before finally fracturing.
- Electroless Nickel Plating
- A process in which nickel coating is applied to a surface in a controlled chemical reduction. Electrons used are not supplied electrically, but by a chemical reducing agent.
- Electroplating
- A process by which metal ions are attracted to a solid metal electrode. As the ions bind to the surface of the metal, they become a thin coating, which forms a protective layer to prevent corrosion.
- Extractive Metallurgy
- The process of purifying and recycling metal that was extracted from ore.
- Ferromagnetic
- It is the most familiar form of magnetism. Permanent magnets are ferromagnetic, and so are the metals that are attracted to them, such as nickel.
- Hydride
- Any binary compound of hydrogen and another element.
- Malleable
- The characteristic of some metals, meaning they have the ability to be shaped or formed by applying pressure.
- Non-ferrous
- A type of metal which does not contain iron.
- Oxidation
- The reaction in which oxygen is added and causes the removal of electrons from the reactant.
- Superalloy
- An alloy with a base element of nickel, nickel-iron or cobalt, which has corrosion resistance, ability to withstand high temperatures, mechanical strength and good surface stability.

